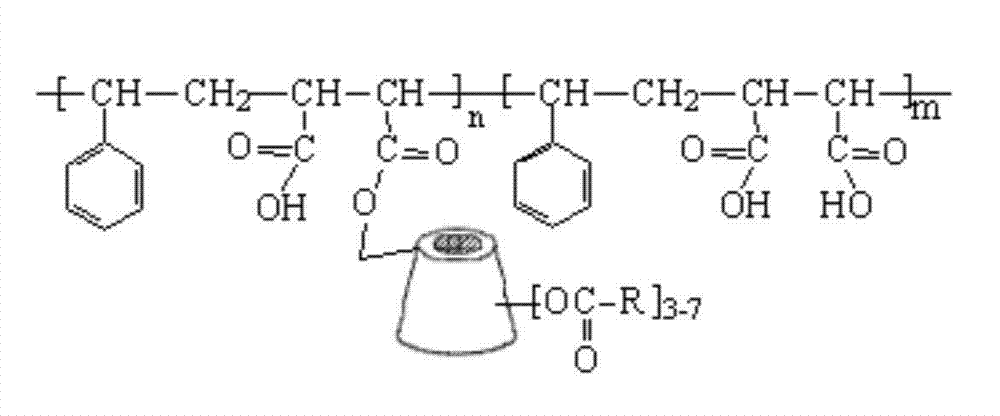
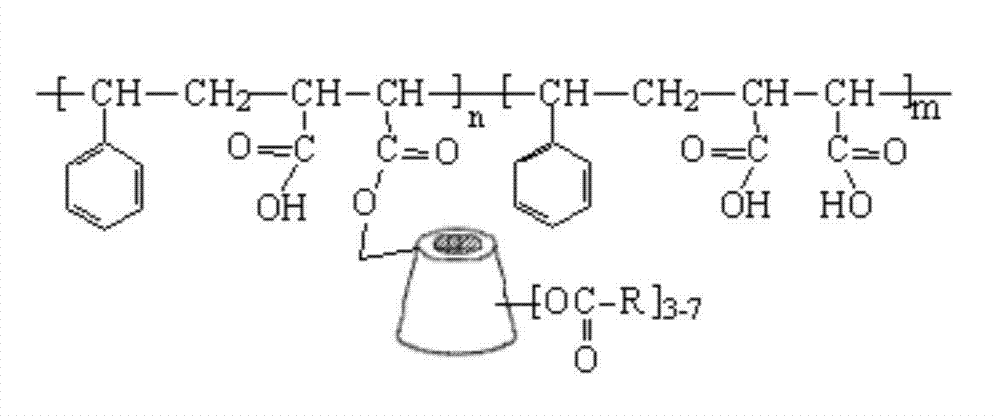
Cyclodextrin immobilized macromolecular polymer and preparation method and application thereof
A high-molecular polymer and cyclodextrin technology, applied in chemical instruments and methods, other chemical processes, adsorption water/sewage treatment, etc., can solve problems such as non-absorbable materials, and achieve simple preparation methods, easy industrialization, and raw material sources Wide and cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put 3.0g styrene-maleic anhydride copolymer and 25.0g dry β-cyclodextrin in 60ml N,N-dimethylformamide, react at 50°C for 10h, remove most of the solvent under reduced pressure, and use 30°C Warm water washes the residue repeatedly to obtain β-cyclodextrin-immobilized styrene-maleic anhydride copolymer.
[0021] Dissolve 5.0 g of the solid substance in 80 ml of DMF, add 3.0 g of chloroacetyl chloride, and then raise the temperature to 90° C. for 24 hours. Most of the solvent was distilled off under reduced pressure, and the residue was washed with warm water at 50°C to obtain a cyclodextrin-immobilized polymer.
Embodiment 2
[0023] Put 3.0g styrene-maleic anhydride copolymer and 25.0g dry carboxymethyl-β-cyclodextrin in 60ml N,N-dimethylformamide, react at 70°C for 24h, remove most of the solvent under reduced pressure, Repeated washing of the detergent residue with warm water at 30°C yielded carboxymethyl-β-cyclodextrin immobilized styrene-maleic anhydride copolymer.
[0024] Dissolve 5.0 g of the solid substance in 80 ml of DMF, add 3 g of propionyl chloride dropwise, and raise the temperature to 70° C. for 24 h. Most of the solvent was distilled off under reduced pressure, and the residue was washed with warm water at 50°C to obtain a cyclodextrin-immobilized polymer.
Embodiment 3
[0025] Example 3: Adsorption of cyclodextrin-immobilized polymers to organic basic dyes
[0026] Put 0.025g of cyclodextrin-immobilized polymer in 100ml of a certain concentration of organic basic dye basic fuchsin (methylene blue) solution, seal it, and stir at a certain temperature for 12h. Centrifuge to remove the adsorbent, measure the concentration of the solution before and after adsorption with a UV-Vis spectrophotometer, and calculate the adsorption amount and adsorption rate.
[0027] The results show that: at 35°C, the saturated adsorption capacity of the adsorbent for basic fuchsin and methylene blue calculated by the Langmuir isotherm adsorption equation is 170 mg.g -1 and 200 mg.g -1 ; For an initial concentration of 50 mg.L -1 The following basic dye removal rate is above 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com