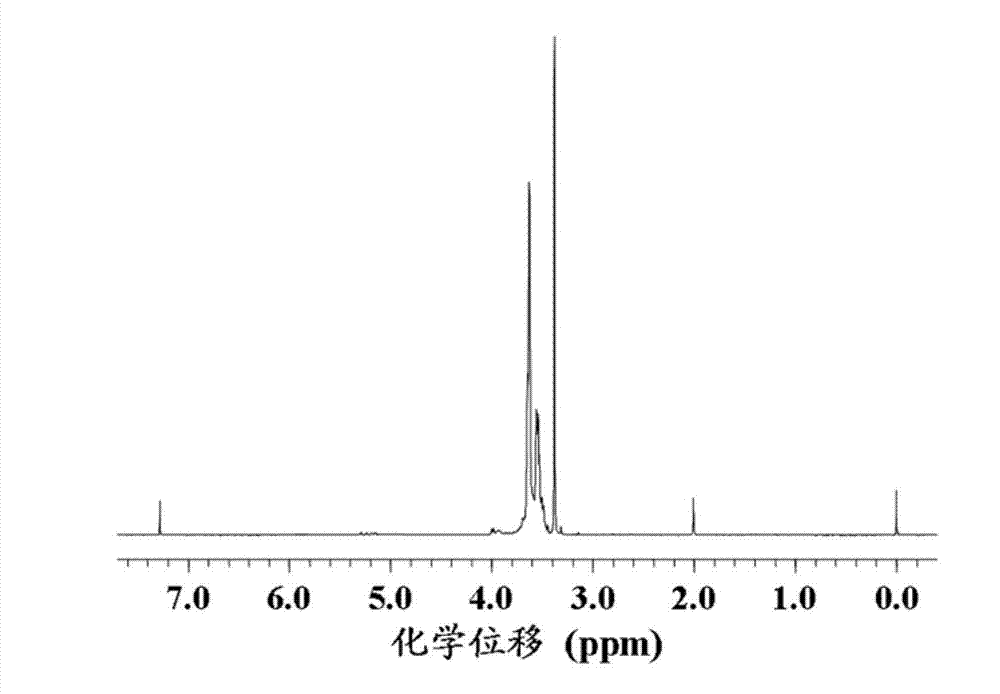
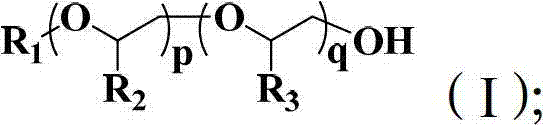
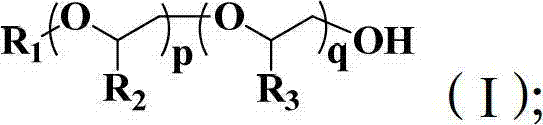
Polyether block copolymer and preparation method thereof
A polyether block and copolymer technology, applied in the field of polymers, can solve the problems of no modifiable side groups, limited applications, and can not provide hydrophobic groups, etc., and achieves high use value and good cell targeting. The effect of sex, wide application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The present invention provides a kind of preparation method of polyether block copolymer described in above-mentioned technical scheme, comprises the following steps:
[0054] a) performing a first polymerization reaction with an anionic initiator and a first polymerizable monomer in an anhydrous solvent to obtain a polyether homopolymer;
[0055] b) performing a second polymerization reaction on the polyether homopolymer obtained in step a) and the second polymerizable monomer to obtain a polyether block copolymer;
[0056] Described anionic initiator is prepared according to the following method:
[0057] Under the atmosphere of inert gas, react the condensed ring aromatic hydrocarbon with 1~5 benzene ring number and alkali metal in anhydrous solvent to obtain the first reaction product;
[0058] reacting the first reaction product with an alcohol compound having 1 to 10 carbon atoms to obtain an anionic initiator;
[0059] The first polymerizable monomer and the se...
Embodiment 1
[0075] Under the atmosphere of dry inert gas, add 5 g (0.039 mol) of naphthalene, 1.52 g (0.039 mol) of potassium and 100 mL of anhydrous tetrahydrofuran to a dry and clean reaction flask, react at 15 °C for 5 h, then add 3-allyl alcohol 2.26g (0.039mol), reacted at 15°C for 24h to obtain an anionic initiator.
Embodiment 2
[0077] Under the atmosphere of dry inert gas, add 5g (0.039mol) of naphthalene, 1.52g (0.039mol) of potassium and 100mL of anhydrous tetrahydrofuran to a dry and clean reaction flask, react at 25°C for 5h, then add 3-allyl alcohol 2.26g (0.039mol), reacted at 25°C for 24h to obtain an anionic initiator.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com