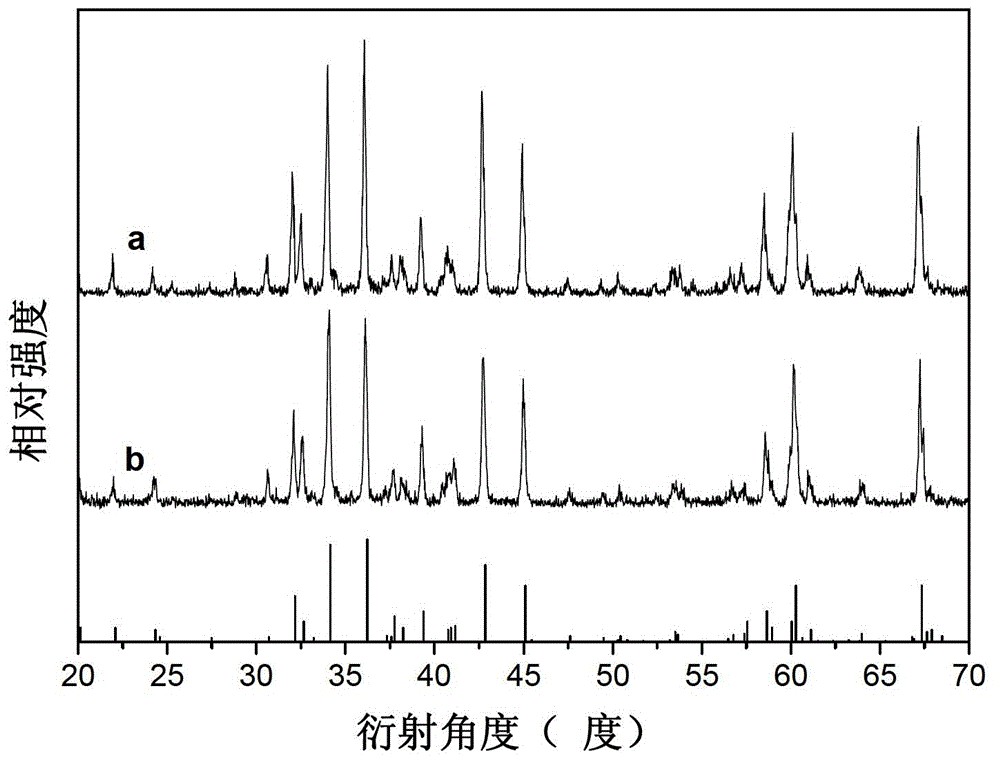
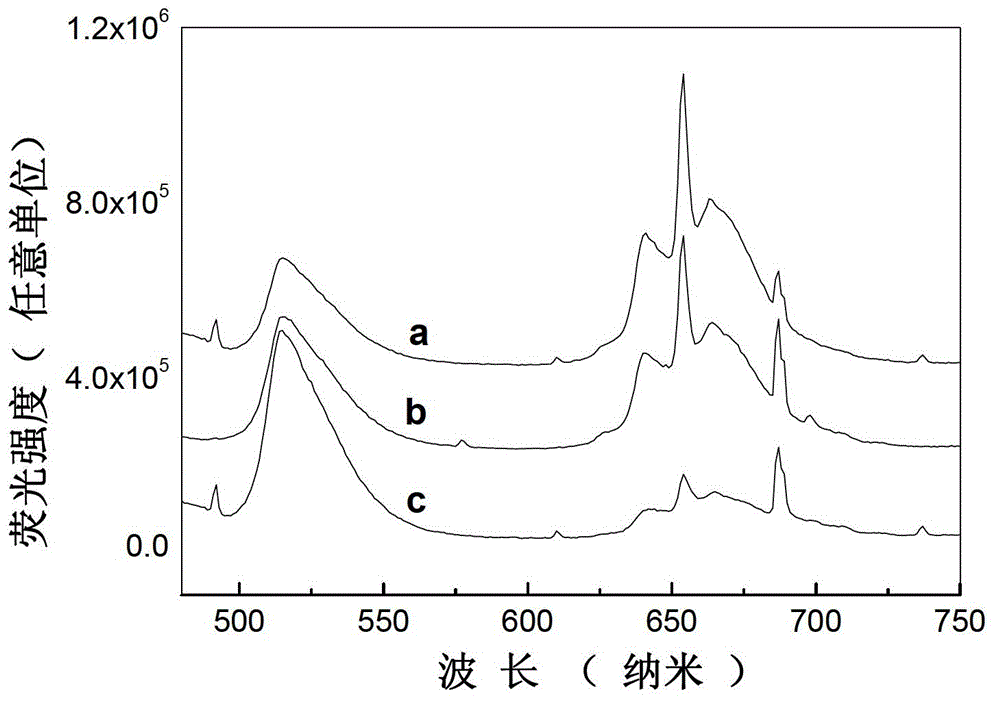
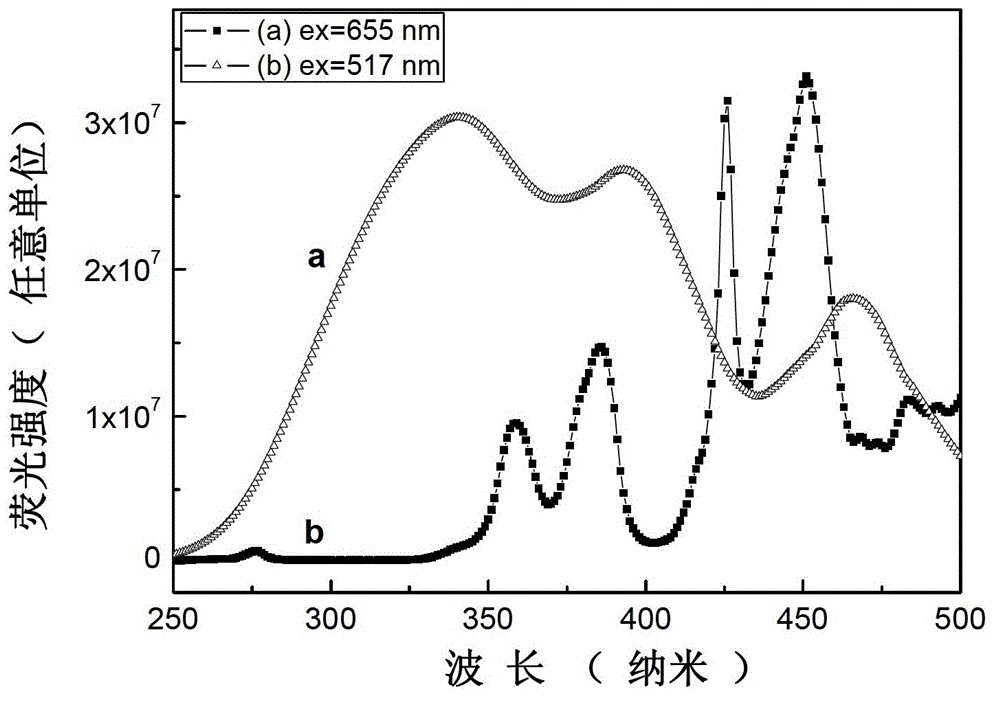
Light color adjustable valence alternation manganese ion doped aluminate luminescent material and preparation method thereof
A technology of luminescent materials and manganese ions, which is applied in luminescent materials, chemical instruments and methods, sustainable buildings, etc., can solve the problems of expensive raw materials, high cost, and unfavorable large-scale process production, and achieve low prices and ease the shortage of supply. The pressure, easy to operate the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] According to CaCO 3 : Al 2 o 3 :MnO 2 : Bi(NO 3 ) 3 ·5H 2 O=0.99:12:0.5%:0.01 molar ratio, accurately weigh 0.9900g (0.0099mol) of solid calcium carbonate CaCO with an electronic balance 3 (analytical pure), 6.1176g (0.06mol) solid alumina Al 2 o 3 (analytically pure), 0.0043g (0.00005mol) solid manganese dioxide MnO 2 (analytically pure), and 0.0485g (0.0001mol) bismuth nitrate Bi(NO 3 ) 3 ·5H 2 O (analytically pure), fully grind in an agate mortar for 40 minutes, and mix well to obtain a mixture. Under air conditions, put the mixture in a muffle furnace at 800°C for 5 hours, take it out after cooling, and grind it again for 10 minutes to obtain a precursor, put the precursor in the air at 1500°C for 3 hours, and take it out after cooling. Instant product. Using the Fluoromax-4 fluorescence spectrometer (HORIBA Jobin Yvon Inc.) to study its luminescence properties, it was observed that the product emitted Mn under the excitation of a 396nm xenon lamp 2+ T...
Embodiment 2
[0034] According to CaCO 3 : Al 2 o 3 :MnO 2 : Bi(NO 3 ) 3 ·5H 2 O=0.97:12:0.5%:0.03 molar ratio, accurately weigh 0.9700g (0.0097mol) solid calcium carbonate CaCO with an electronic balance 3 (analytical pure), 6.1176g (0.06mol) solid alumina Al 2 o 3 (analytically pure), 0.0043g (0.00005mol) solid manganese dioxide MnO 2 (analytical pure), and 0.1454g (0.0003mol) bismuth nitrate Bi(NO 3 ) 3 ·5H 2 O (analytically pure), fully grind in an agate mortar for 30 minutes, and mix well to obtain a mixture. Under air conditions, put the mixture in a muffle furnace at 900°C for 5 hours, take it out after cooling, and grind it again for 10 minutes to obtain a precursor, put the precursor in the air at 1300°C for 3 hours, and take it out after cooling. Instant product. Using the Fluoromax-4 fluorescence spectrometer (HORIBA Jobin Yvon Inc.) to study its luminescence properties, it was observed that the product emitted Mn under the excitation of a 396nm xenon lamp 2+ The wa...
Embodiment 3
[0036] According to CaCO 3 : Al 2 o 3 :MnO 2 : Bi(NO 3 ) 3 ·5H 2 O=0.93:12:0.5%:0.07 molar ratio, accurately weigh 0.9300g (0.0093mol) solid calcium carbonate CaCO with an electronic balance 3 (analytical pure), 6.1176g (0.06mol) solid alumina Al 2 o 3 (analytically pure), 0.0043g (0.00005mol) solid manganese dioxide MnO 2 (analytically pure), and 0.33915g (0.0007mol) bismuth nitrate Bi(NO 3 ) 3 ·5H 2O (analytically pure), fully grind in an agate mortar for 10 minutes, and mix well to obtain a mixture. Under air conditions, put the mixture in a muffle furnace at 1000°C for 5 hours, take it out after cooling, and grind it again for 20 minutes to obtain a precursor, put the precursor in the air at 1300°C for 3 hours, and take it out after cooling. Instant product. Fluoromax-4 fluorescence spectrometer (HORIBA Jobin Yvon Inc.) was used to study its luminescent performance. Under the excitation of 396nm xenon lamp, the product emitted strong green light at 500-550nm a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com