Target degradation Bcr-Abl protein reagent and application of target degradation Bcr-Abl protein reagent in preparing Philadelphia chromosome positive tumor treatment medicine
A Philadelphia chromosome and bcr-abl technology, which is applied to a reagent for targeting degrading Bcr-Abl protein and its application in the preparation of a Philadelphia chromosome positive tumor treatment drug, and achieves the effects of inhibiting growth, good curative effect and difficult reversal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Rubescensine A efficiently, rapidly and irreversibly degrades Bcr-Abl protein in Ph-positive tumor cells
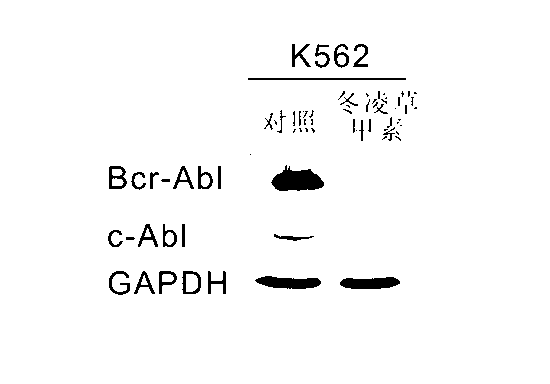
[0034] We tested the effect of oridonin on Bcr-Abl fusion protein in the Ph positive tumor cell line K562. Each cell is divided into 3x10 5 Cells / mL were inoculated on cell culture plates, cultured for 24 hours to the logarithmic growth phase, and then treated with 20 μM oridonin and drug solvent DMSO. After 24 hours of induction, the cells were collected and the total protein was extracted by SDS method for Western blot detection. The results showed that compared with the control DMSO treatment, Rubescensin A treatment significantly reduced the Bcr-Abl and c-Abl protein levels in K562 cells, and almost completely degraded them, while the level of the internal control GAPDH protein had no significant change ( figure 1 ). It shows that Rubescensine A can specifically degrade the oncoprotein members in the Bcr-Abl protein family in K562 cells.
[0035...
Embodiment 2
[0039] Example 2 Rubescensine A targets ubiquitination to degrade Bcr-Abl protein
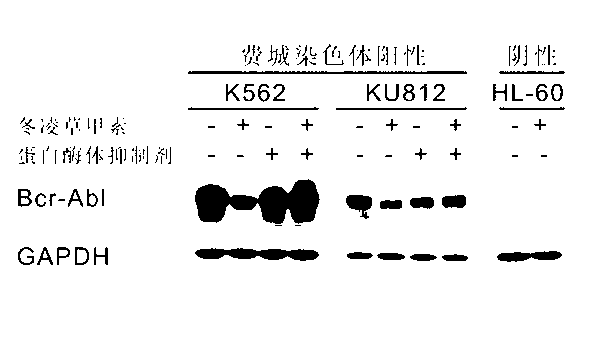
[0040] K562 cells were inoculated, and after 24 hours of culture, three proteasome inhibitors MG-132 (M, 10 μM), ALLN (A, 100 μM) or Lactacystin (L, 25 μM) were added for pretreatment for 2 hours, and then 20 μM winter Induction by iconidin. After 2 hours, the cells were collected and the total protein of the cells was extracted by SDS method for western blot detection. MG-132, ALLN and Lactacystin could completely inhibit the degradation of Bcr-Abl and c-Abl proteins by oridonin ( Figure 6 ), indicating that the degradation of Bcr-Abl and c-Abl by Rubescensin A depends on the ubiquitin-proteasome pathway.
[0041] K562 cells and KU812 cells were inoculated, cultured for 24 hours, and then pretreated with proteasome inhibitor MG-132 for 2 hours, and then induced with 20 μM oridonin. After 2 hours, the cells were collected and the total protein of the cells was extracted by SDS meth...
Embodiment 3
[0042] Example 3 Rubescensine A inhibits the growth of Ph-positive tumor cells and induces irreversible apoptosis
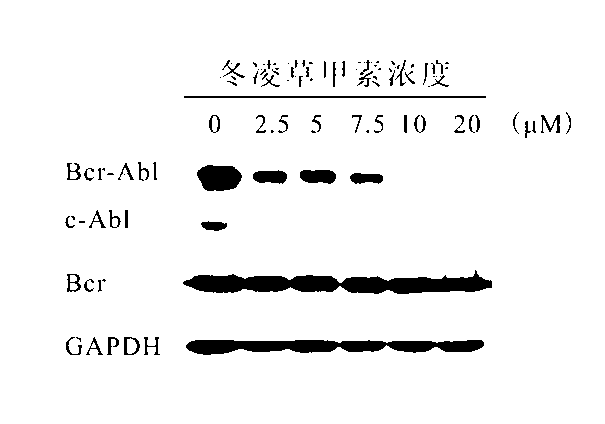
[0043] K562 cells were inoculated and treated with 0-20 μM oridonin after 24 hours of culture. After 24 hours of induction, the cell survival rate was detected by MTT, and the cells were collected to detect cell apoptosis by AnnexinV-FITC / PI staining. Under the treatment of different concentrations of oridonin, K562 cells and KU812 cells experienced different degrees of growth inhibition and apoptosis, and showed an obvious dose-dependent effect. 20 μM Oridonin A treatment for 24 hours, the growth of K562 cells and KU812 cells was significantly inhibited ( Figure 7 A, Figure 8 A), the survival rates were only 32% and 21%, respectively, and significant apoptosis occurred, the apoptosis rate was greater than 50% ( Figure 7 B, Figure 8 B).
[0044] K562 cells were inoculated and treated with 0-20 μM oridonin after 24 hours of culture. After 24 hours of i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com