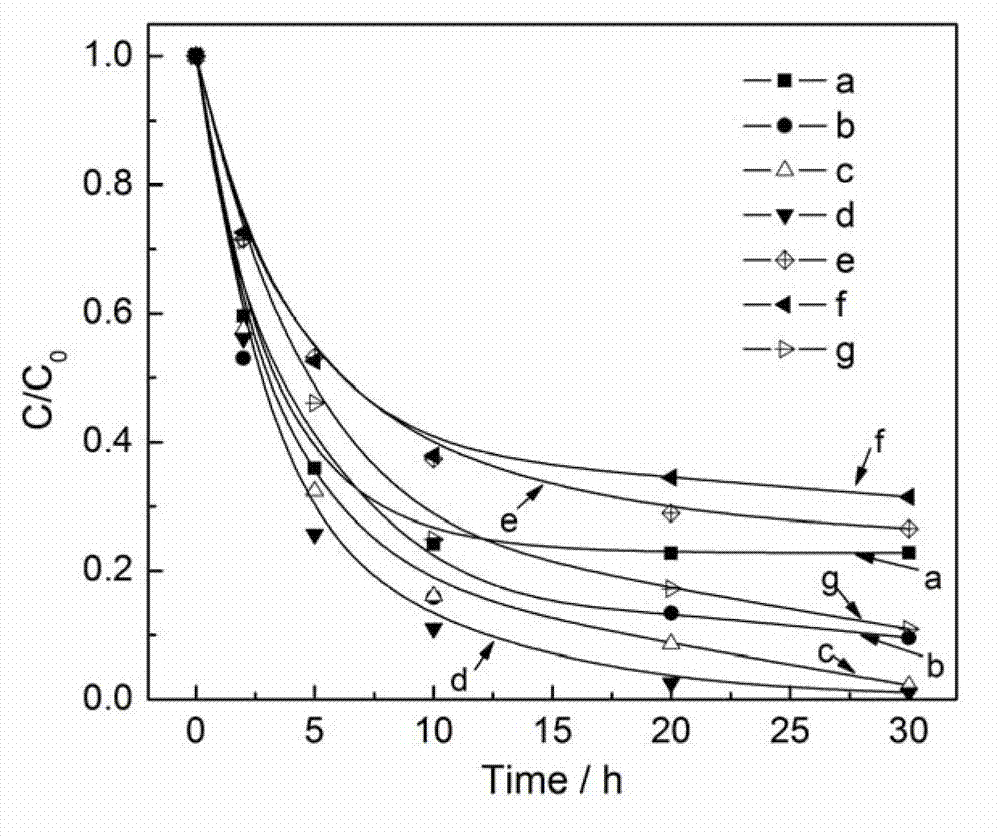
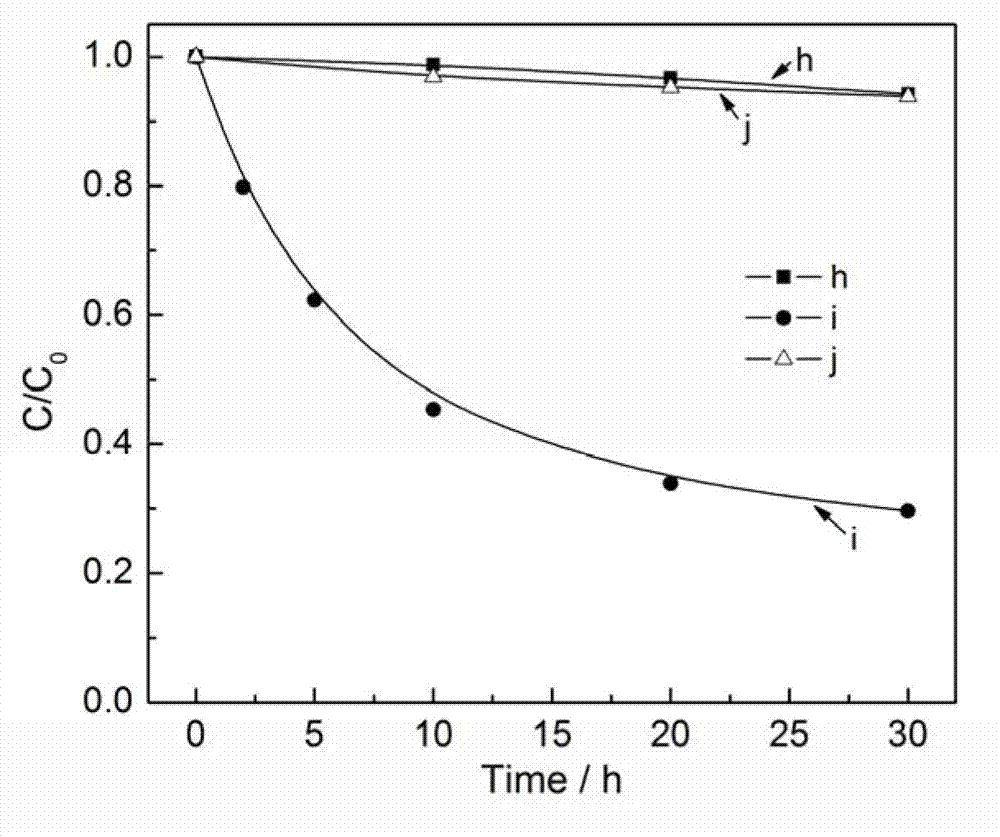
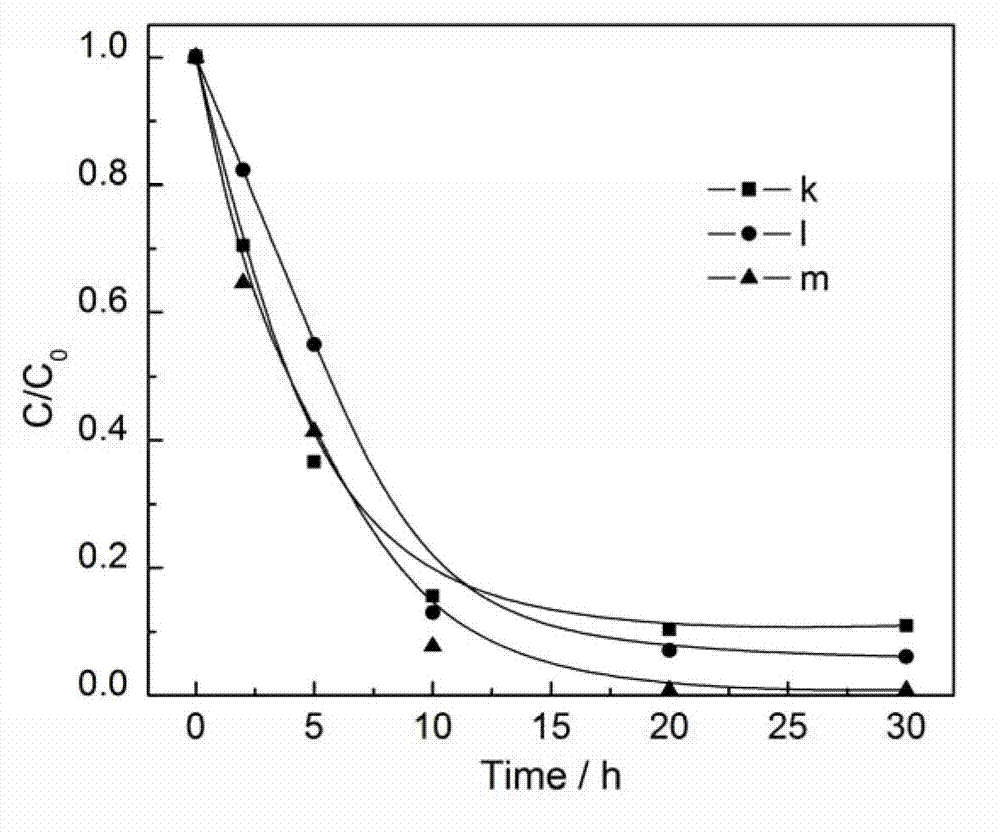
Photocatalyst for degrading organic pollutant and preparation method thereof
A technology of organic pollutants and photocatalysts, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem of low photocatalytic activity, and achieve stable photocatalytic degradation performance and good visible light. Degradation activity, effect of high visible light responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh 1.052g of samarium oxide, add 3mL of concentrated nitric acid and 12mL of deionized water, stir and dissolve to obtain a samarium nitrate solution. Weigh 0.706g of ammonium metavanadate, add 6mL concentrated ammonia water and 24mL deionized water, stir until dissolved, then add samarium nitrate solution drop by drop, adjust the mixture pH=7, a white precipitate is formed, continue to stir and age for 1h to obtain the mixture Ⅰ . Weigh 0.362 g of silver nitrate and dissolve it in 10 mL of deionized water, add it dropwise to I, and continue stirring for 30 min to obtain mixture II. Then weigh 0.220g of sodium bromide and dissolve it in 10mL of deionized water, add it dropwise to II, stir and age for 5 hours, then centrifuge and wash, dry the obtained solid in an oven at 120°C, grind it, and finally roast it at 500°C After cooling for 2 hours, 0.353AgBr / SmVO calcined at 500°C was obtained 4 catalyst.
Embodiment 2
[0023] Weigh 0.920 g of samarium oxide, add 3 mL of concentrated nitric acid and 12 mL of deionized water, stir and dissolve to obtain a samarium nitrate solution. Weigh 0.618g of ammonium metavanadate, add 6mL of concentrated ammonia water and 24mL of deionized water, stir until dissolved, then add samarium nitrate solution drop by drop, adjust the pH of the mixture to 7, a white precipitate is formed, continue to stir and age for 1h to obtain the mixture Ⅰ . Weigh 0.542 g of silver nitrate and dissolve it in 10 mL of deionized water, add it dropwise to I, and continue stirring for 30 min to obtain mixture II. Then weigh 0.328g of sodium bromide, dissolve it in 10mL of deionized water, add it dropwise to II, stir and age for 5 hours, then centrifuge and wash, dry the obtained solid in an oven at 120°C, grind it, and finally roast it at 500°C After cooling for 2 hours, 0.605AgBr / SmVO calcined at 500°C was obtained 4 catalyst.
Embodiment 3
[0025] Weigh 0.788g of samarium oxide, add 3mL of concentrated nitric acid and 12mL of deionized water, stir and dissolve to obtain a samarium nitrate solution. Weigh 0.530g of ammonium metavanadate, add 6mL of concentrated ammonia water and 24mL of deionized water, stir until dissolved, then add samarium nitrate solution drop by drop, adjust the pH of the mixture to 7, and form a white precipitate, continue to stir and age for 1h to obtain the mixture Ⅰ . Weigh 0.724 g of silver nitrate and dissolve it in 10 mL of deionized water, add it dropwise to I, and continue stirring for 30 min to obtain mixture II. Then weigh 0.438g of sodium bromide, dissolve it in 10mL of deionized water, add it dropwise to II, stir and age for 5 hours, then centrifuge and wash, dry the obtained solid in an oven at 120°C, grind it, and finally roast it at 500°C After cooling for 2 hours, 0.942AgBr / SmVO calcined at 500°C was obtained 4 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com