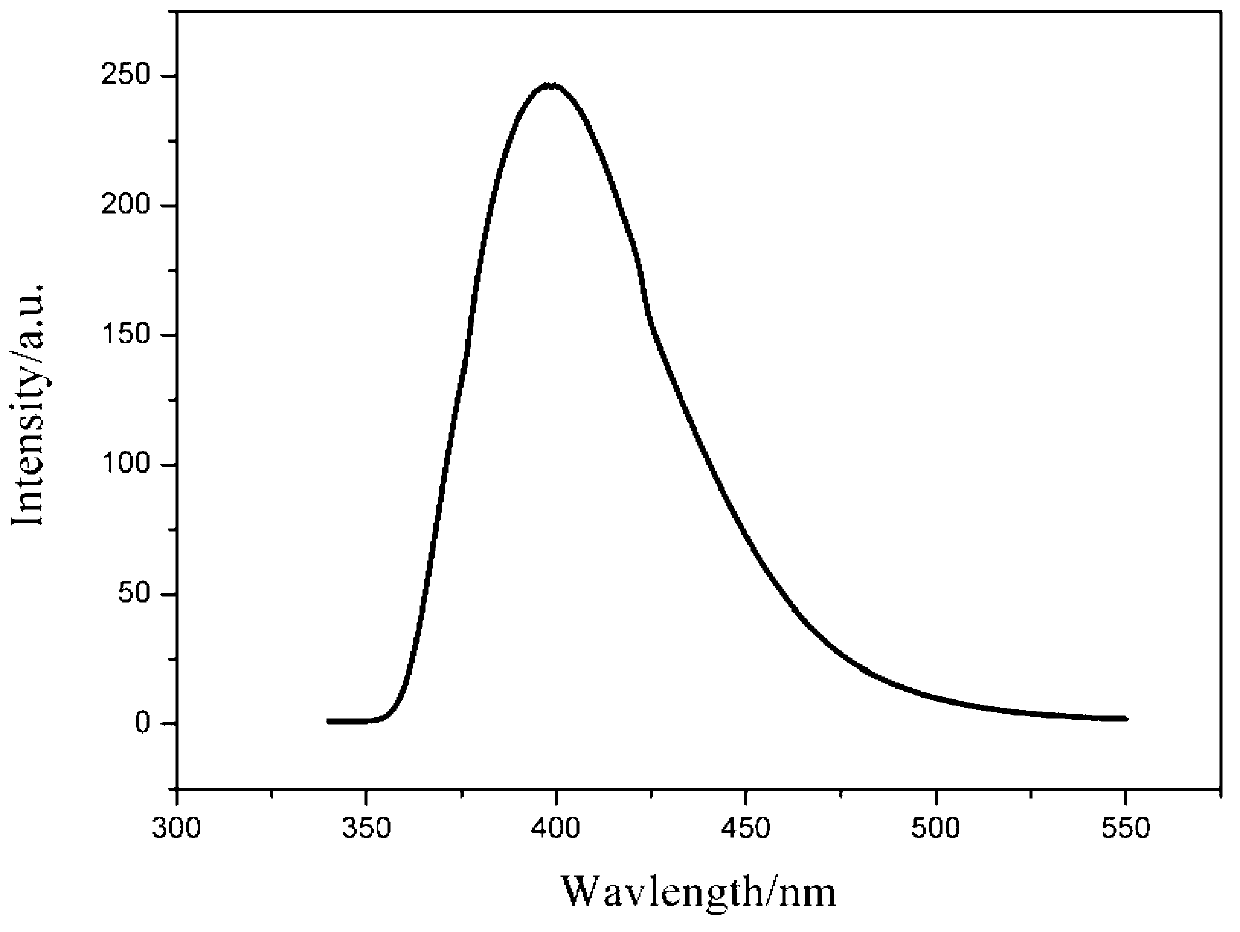
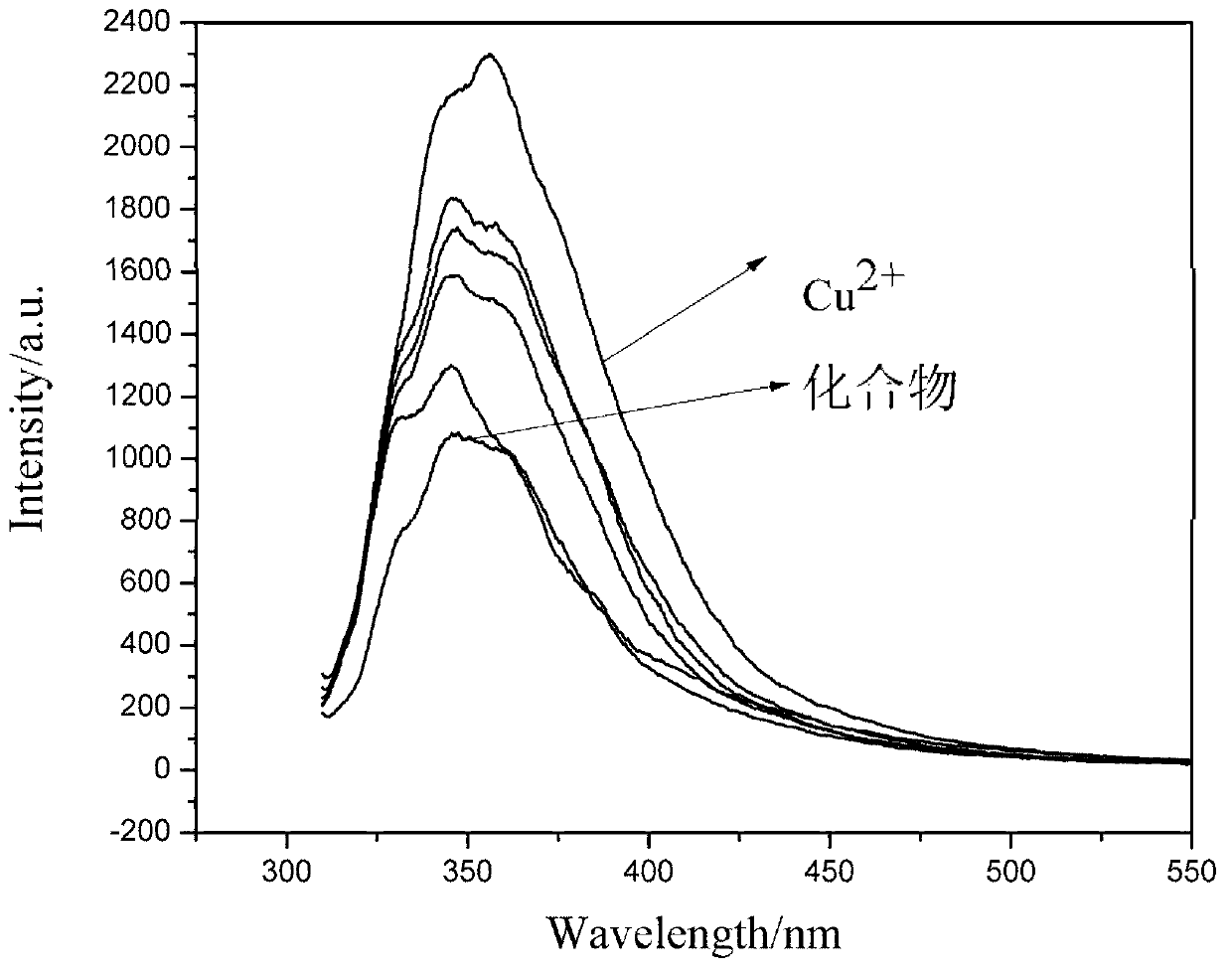
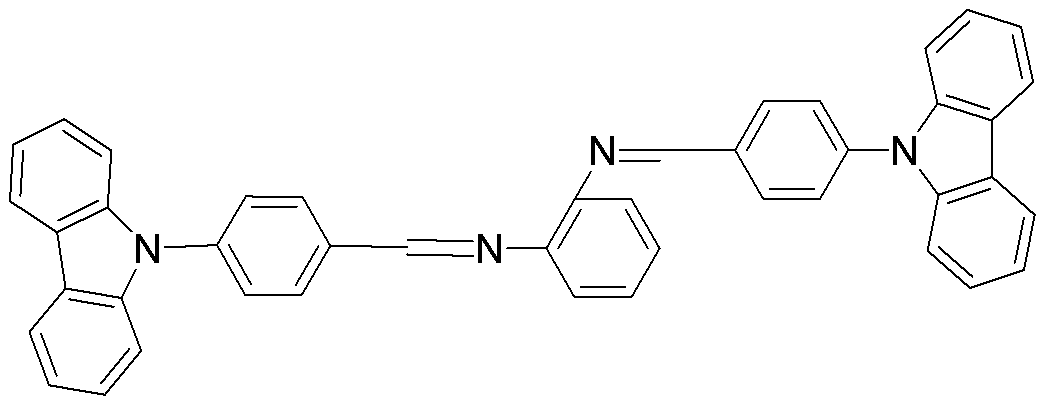
Carbazole benzaldehyde o-phenylenediamine bis-schiff base and preparation method thereof
A technology of carbazole benzaldehyde o-phenylenediamine and carbazole benzaldehyde phenylenediamine, applied in the field of carbazole benzaldehyde o-phenylenediamine bis-Schiff base and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 2.16g (0.008mol) of carbazole benzaldehyde and dissolve it in 30ml of acetone, start stirring until the carbazole benzaldehyde is completely dissolved, add 0.1g (0.001mol) of o-phenylenediamine, add 3d glacial acetic acid, and control the temperature at 56 ℃, reacted for 12 hours, and obtained yellow Schiff base powder, which was recrystallized from ethanol, and dried in vacuum to obtain carbazole benzaldehyde o-phenylenediamine bis-Schiff base, yield: 65%.
[0036] P1 (reactants: carbazole benzaldehyde, o-phenylenediamine)
[0037]
[0038] 1H NMR (d-DMSO) δ: 8.48(d, 4H, J=7.6), 8.28(d, 4H, J=7.6), 7.85(d, 4H, J=8.0), 7.72(s, 2H), 7.59 (d, 2H, J=7.6), 7.50(m, 6H), 7.32(t, 4H, J=7.2), 7.24(t, 4H, J=7.8).
[0039] Elemental Analysis: C 44 h 30 N 4 : %C85.97(85.99);%H4.92(4.89);9.10(9.11) The measured values are in the brackets;
[0040] MS (ESP) m / z: 614.25 (M).
Embodiment 2
[0042] Weigh 1.08g (0.004mol) of carbazole benzaldehyde and dissolve it in 40ml of ethanol, start stirring until the carbazole benzaldehyde is completely dissolved, add 0.1g (0.001mol) of o-phenylenediamine, add 8d formic acid, and control the temperature at 75°C , and reacted for 8 hours to obtain a yellow Schiff base powder. The powder was recrystallized from dichloromethane and dried in vacuo to obtain carbazole benzaldehyde o-phenylenediamine bis-Schiff base. Yield: 70%. For the structural characterization data of the compound, see Example 1.
Embodiment 3
[0044] Weigh 2.16g (0.008mol) of carbazole benzaldehyde and dissolve it in 60ml of methanol, start stirring until the carbazole benzaldehyde is completely dissolved, add 0.3g (0.003mol) of o-phenylenediamine, add 5d formic acid, and control the temperature at 65°C , and reacted for 2 hours to obtain a yellow Schiff base powder. The powder was recrystallized from chloroform and dried in vacuum to obtain carbazole benzaldehyde o-phenylenediamine bis-Schiff base. The yield: 67%. For the structural characterization data of the compound, see the examples 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com