Tetraphenylethylene-phenanthroimidazole derivative and crystal, preparation method and application thereof
A technology of phenanthroimidazole and tetraphenylethylene, applied in the field of luminescent materials, can solve problems such as poor thermal stability, low luminous intensity, and concentration quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
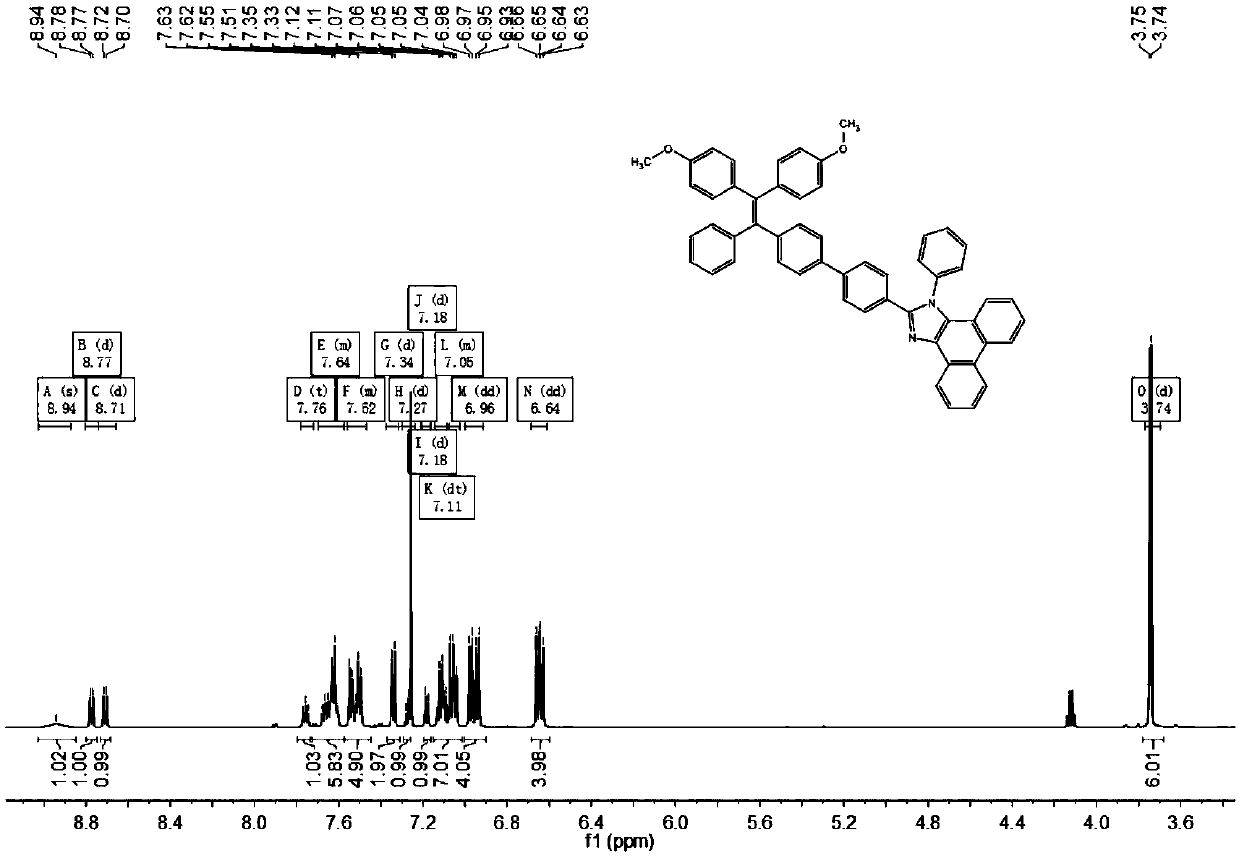
[0082] (1) Preparation of formula (II) compound
[0083] Weigh 1.82g 4,4-dihydroxy-benzophenone, 6.5g 4-bromo-benzophenone and 6.53g zinc powder in a 250mL round-bottomed flask, add 100mL of tetrahydrofuran to dissolve to obtain a mixed solution; Under the protection of nitrogen, perform magnetic stirring, cool to 0°C, inject 5.6ml of titanium tetrachloride, stir at -10°C for 30min, heat to reflux at 90°C for 20h, cool to room temperature, and add 60ml of dilute hydrochloric acid to quench the reaction. After purification by column chromatography, the eluent was n-hexane / ethyl acetate, and 3.05 g of the compound of formula (II) was isolated as a white solid.
[0084] The reaction equation is as follows:
[0085]
[0086] (2) Preparation of formula (Ⅲ-1) compound
[0087] Weigh 1.2g of (II) compound, 1.54g of methyl iodide and 1.9g of potassium carbonate in a 100ml round bottom flask, add 50ml of N,N dimethylformamide, and carry out magnetic stirring under the protection o...
Embodiment 2
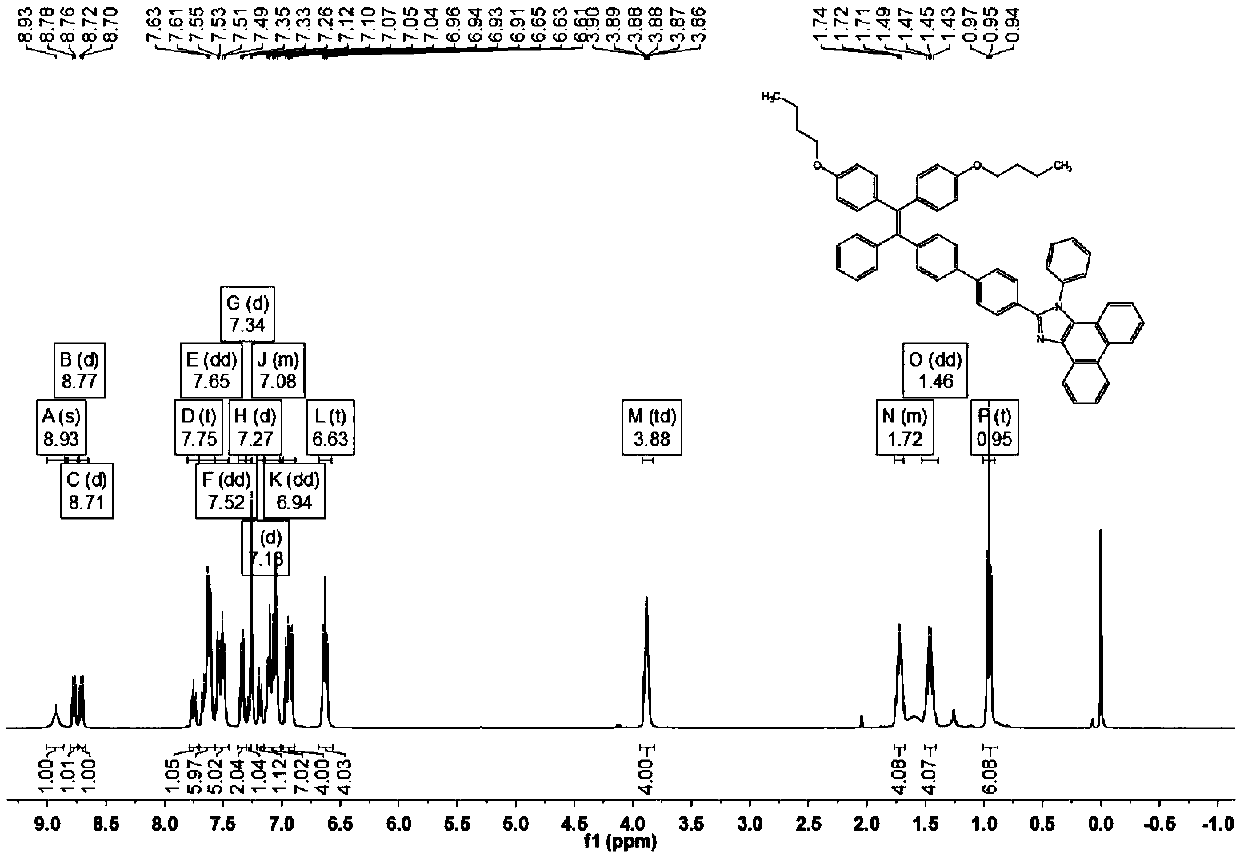
[0099] (1) The preparation of the compound of formula (II) is the same as in Example 1
[0100] (2) Preparation of formula (Ⅲ-2) compound
[0101] Weigh 1.2g of the compound of formula (II), 1.54g of n-bromobutane and 1.9g of potassium carbonate in a 100ml round bottom flask, add 50ml of N,N dimethylformamide, carry out magnetic stirring under the protection of nitrogen, and heat to temperature 70°C, reacted for 15 hours, and cooled to room temperature. After purification by column chromatography, the eluent was n-hexane / dichloromethane, and 1.08 g of the compound of formula (III-2) was isolated as a yellow oil.
[0102] The reaction equation is as follows:
[0103]
[0104] (3) preparation of formula (Ⅴ-2) compound
[0105] Weigh 0.94g formula (Ⅲ-2) compound, 0.5g formula (Ⅳ-1) compound 4-formylphenylboronic acid, 1.52g potassium carbonate and 0.12g tetrakistriphenylphosphine palladium in a 100ml round bottom flask, add 40ml Tetrahydrofuran and 10ml of water were magne...
Embodiment 3
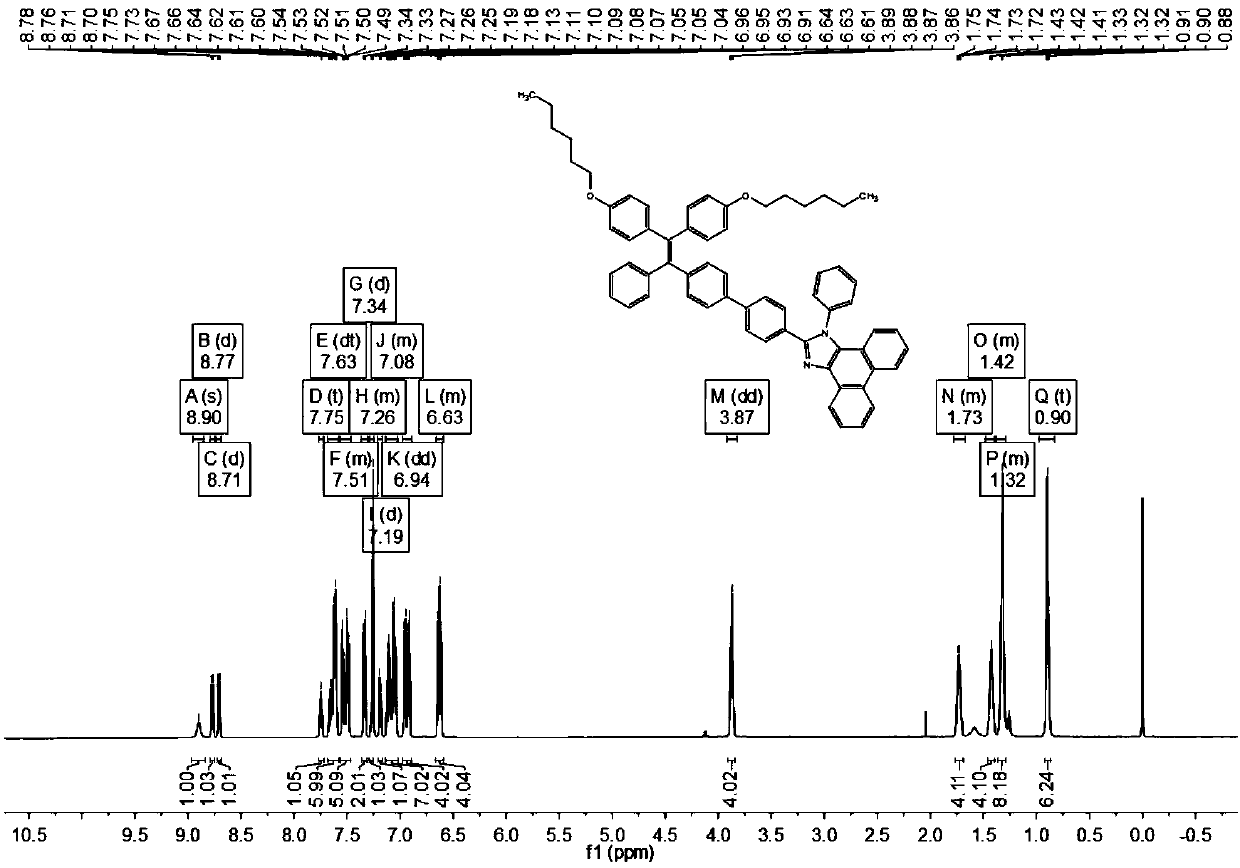
[0113] (1) The preparation of the compound of formula (II) is the same as in Example 1
[0114] (2) Preparation of formula (Ⅲ-3) compound
[0115] Weigh 1.2g of the compound of formula (II), 1.54g of n-bromohexane and 1.9g of potassium carbonate in a 100ml round bottom flask, add 50ml of N,N dimethylformamide, and carry out magnetic stirring under the protection of nitrogen, heating to temperature 70°C, reacted for 15 hours, and cooled to room temperature. After purification by column chromatography, the eluents were n-hexane and dichloromethane, and 1.08 g of the compound of formula (III-3) was isolated as a yellow oil.
[0116] The reaction equation is as follows:
[0117]
[0118] (3) preparation of formula (Ⅴ-3) compound
[0119] Weigh 0.94g formula (Ⅲ-3) compound, 0.5g formula (Ⅳ-1) compound 4-formylphenylboronic acid, 1.52g potassium carbonate and 0.12g tetrakistriphenylphosphine palladium in a 100ml round bottom flask, add 40ml Tetrahydrofuran and 10ml of water w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com