Acid-addition salt of Prasugrel, preparation method and application
A technology of benzenesulfonate and hydroxybenzenesulfonate, which is applied in the preparation of sulfonate, medical preparations containing active ingredients, pharmaceutical formulations, etc., and can solve the problems of properties such as solubility, stability, and biological activity. Detailed evaluation, no advantages and other problems were found, to achieve the effect of simple and easy preparation method, good stability and safety, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
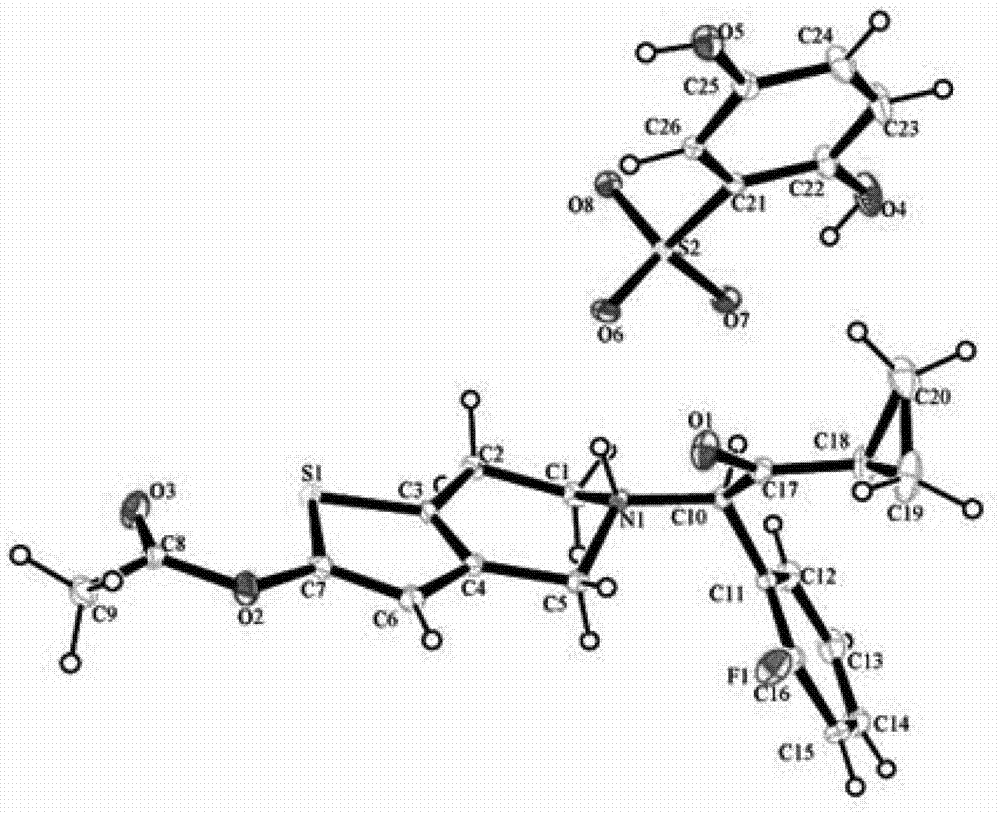
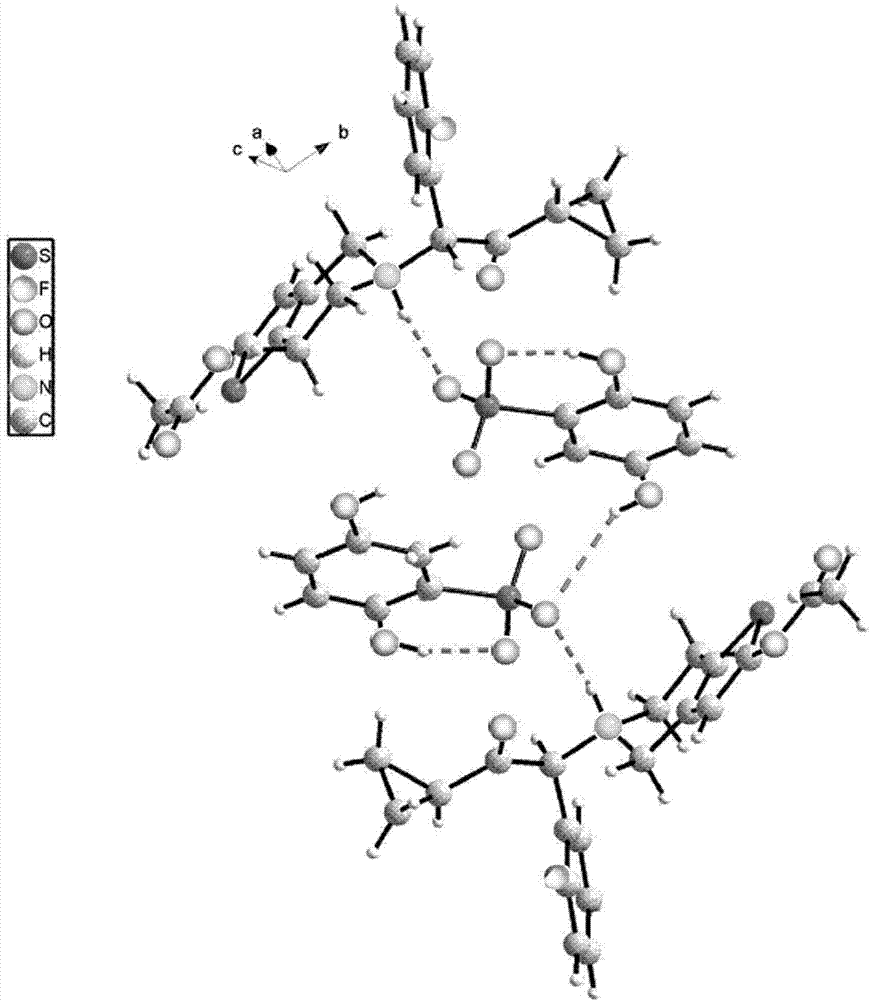
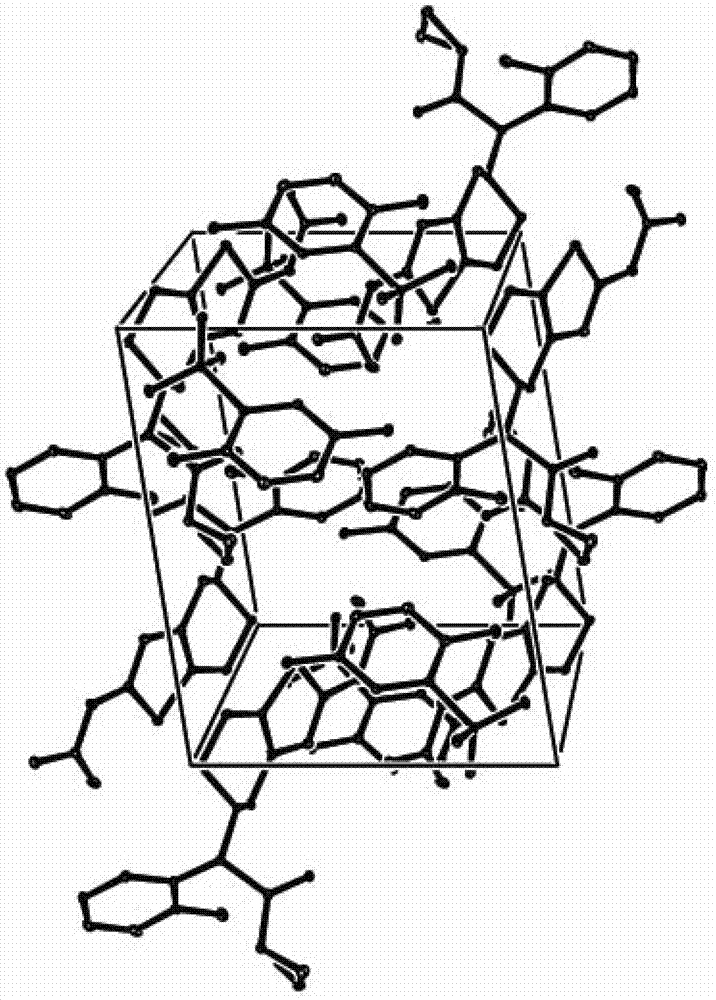
[0061] Example 1: Synthesis of prasugrel 2,5-dihydroxybenzenesulfonate
[0062] Add prasugrel (17.2g, 0.046mol) and 2,5-dihydroxybenzenesulfonic acid (10.5g, 0.055mol) into a reaction flask filled with acetone (170ml), control the temperature at 30°C, and stir to make it Dissolved to give a clear solution. Concentrate the reaction solution to 1 / 2 volume, let it stand, cool to 0°C, and precipitate a single crystal, which is the 2,5-dihydroxybenzenesulfonate of prasugrel, that is, prasugrel 2,5-dihydroxybenzenesulfonate , weight: 22.2g, yield: 85.6%. The crystal form corresponding to the product is named Form A.
[0063] The following are the characterization data of prasugrel 2,5-dihydroxybenzenesulfonate:
[0064] m.p.175.3-176.5°C.
[0065] IR(KBr) v :3303(O-H),3185,3021(C=C-H),2929(C-H),1751(O-C=O),1700(C=O),1209,1076(SO3-NH + ), 1193(C-F).
[0066] ESI-MS (positive): m / z=374, [M+H] + ;m / z=396,[M+Na] + .
[0067] ESI-MS (negative): m / z=189, [M-H] - .
[0068] 1 ...
Embodiment 2
[0071] Example 2: Synthesis of prasugrel 2,5-dihydroxybenzenesulfonate
[0072] Add prasugrel (17.2g, 0.046mol) and 2,5-dihydroxybenzenesulfonic acid (10.5g, 0.055mol) into a reaction flask filled with acetone (300ml), control the temperature at 10°C, and stir to make it Dissolved to give a clear solution. Concentrate the reaction solution to 1 / 2 volume, let it stand, cool to 10°C, and crystallize, which is 2,5-dihydroxybenzenesulfonate of prasugrel, weight: 21.0g, yield: 81.1%.
[0073] The characterization results of the product are the same as in Example 1.
Embodiment 3
[0074] Example 3: Synthesis of prasugrel 2,5-dihydroxybenzenesulfonate
[0075] Add prasugrel (17.2g, 0.046mol) and 2,5-dihydroxybenzenesulfonic acid (10.5g, 0.055mol) into a reaction flask filled with acetone (100ml), heat to 56°C, stir to dissolve , a clear solution was obtained. Concentrate the reaction solution to 1 / 2 volume, let stand, cool to 25°C, and crystallize, which is 2,5-dihydroxybenzenesulfonate of prasugrel, weight: 21.8g, yield: 83.9%.
[0076] The characterization results of the product are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com