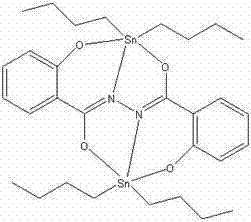
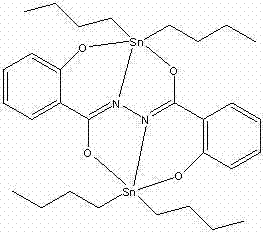
2-hydroxy-N-(2-hydroxybenzoyl) benzo-hydrazide dibutyl tin complex and preparation method and application thereof
A technology of dibutyltin benzohydrazide and hydroxybenzoyl, which is applied in the field of dibutyltin complexes, can solve the problems of biological activity that has not been reported in the literature, and achieve the effects of low cost, high anticancer activity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Dissolve 1.0 mmol 2-hydroxy-N-(2-hydroxybenzoyl)benzohydrazide in 20ml CH 3 OH, then slowly drop 1.0 mmol (Bu) into the solution 4 NH 4 OH (25% aqueous solution), after stirring at room temperature for 0.5 h, 2.0 mmol Bu 2 SnCl 2 Added to the above mixed solution, and continued to stir at room temperature for 18h. After filtration, the filtrate was left standing at room temperature, and the color gradually changed from colorless to red. One month later, red blocky crystals were obtained at 281-283°C. The compound yield was 70.0%.
[0020] Organotin compound of the present invention is through infrared spectrum analysis and nuclear magnetic resonance and X-single crystal diffraction analysis, and result is as follows:
[0021] IR (KBr, cm -1 ): υ = 1637.5 (C=O), 1601.6 (C=N-N=C), 544.5 (Sn-O), 460 (Sn-N).
[0022] Crystallographic data: The compound belongs to the monoclinic crystal system, the space group is P2(1) / c, and the unit cell parameters are: a = 8.8810(...
Embodiment 2
[0024] Dissolve 1.0 mmol 2-hydroxy-N-(2-hydroxybenzoyl)benzohydrazide in 20ml CH 3 OH, and then slowly drop 0.5 mmol (Bu) into the solution 4 NH 4 OH (25% aqueous solution), after stirring at room temperature for 0.5 h, 2.0 mmol Bu 2 SnCl 2 Added to the above mixed solution, and continued to stir at room temperature for 18h. After filtration, the filtrate was left standing at room temperature, and the color gradually changed from colorless to red. One month later, red blocky crystals were obtained at 281-283°C. The compound yield was 52.6%.
Embodiment 3
[0026] Dissolve 1.0 mmol 2-hydroxy-N-(2-hydroxybenzoyl)benzohydrazide in 20ml CH 3 OH, and then slowly drop 1.5 mmol (Bu) into the solution 4 NH 4 OH (25% aqueous solution), after stirring at room temperature for 0.5 h, 2.0 mmol Bu 2 SnCl 2Added to the above mixed solution, and continued to stir at room temperature for 18h. After filtration, the filtrate was left standing at room temperature, and the color gradually changed from colorless to red. One month later, red blocky crystals were obtained at 281-283°C. The compound yield was 66.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com