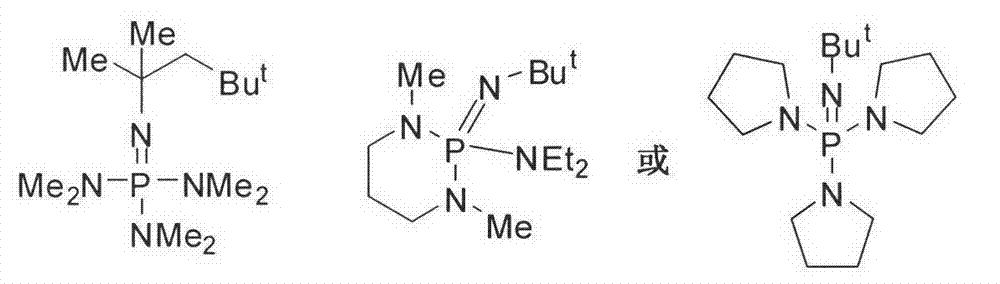
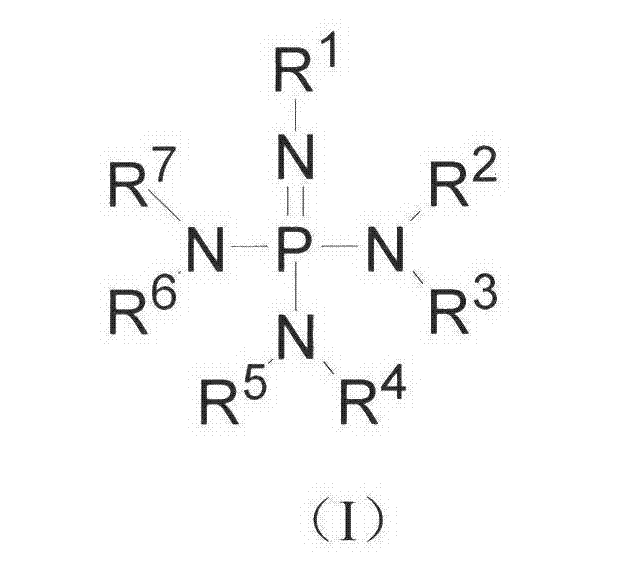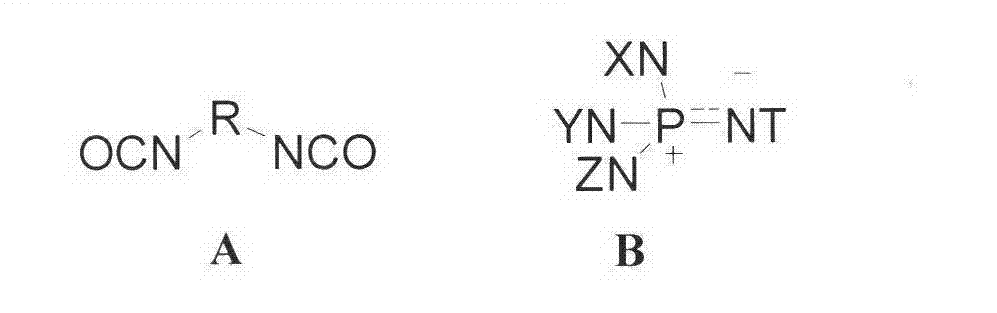Method for preparing polyisocyanate containing formimidoyl oxadiazine diketone
A technology of imino oxadiazine dione and diisocyanate is applied in the field of preparation of polyisocyanate to achieve the effect of improving storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of HDI polyisocyanate: 1500g of newly distilled HDI is first placed in a three-necked flask equipped with a stirring device, the HDI monomer is heated to 60°C, stirred for 1h under reduced pressure to drive out dissolved gas, and then covered with dry nitrogen Under stirring at 60°C, slowly add the above-mentioned catalyst 1 of the present invention, the amount of the catalyst is 100ppm based on the monomeric isocyanate, stir and mix until the temperature rises by 1-2°C as a sign to start the reaction. The mixture was stirred at this temperature for about 2-3 h. When the initial diisocyanate conversion rate reaches 39%, (the conversion rate is determined by gel chromatography) 300ppm benzoyl chloride is added to terminate the reaction, and stirring is continued at 60°C for 1h. The excess monomer HDI was removed in an evaporator to obtain a colorless or light yellow transparent viscous liquid. The component contents of the product are shown in Table 1.
Embodiment 2-6
[0035] The operation of Example 1 was repeated with catalysts 2-6 respectively. The content of the product components is shown in Table 1.
Embodiment 7
[0045] Preparation of HMDI and HDI mixed polyisocyanate: 1000g of newly distilled HDI and 500g of HMDI were first placed in a three-necked flask stirring device, stirred at 60°C for 1h under reduced pressure to drive out dissolved gases, and then covered with dry nitrogen at 80°C Slowly add catalyst 1 under stirring, the amount of catalyst is 150ppm based on monomeric isocyanate, stir and mix until the temperature rises by 1-2°C as a sign to start the reaction. The mixture was stirred at this temperature for about 3-4h. When the initial diisocyanate conversion rate reached 36%, (the conversion rate was determined by gel chromatography). Add 450ppm benzoyl chloride to terminate the reaction, continue stirring at 80°C for 1h, and remove excess monomer HDI and HMDI in a thin-film evaporator at a pressure of 10pa and a temperature of 160°C to obtain a colorless or light yellow transparent viscous liquid. The content of product components is shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com