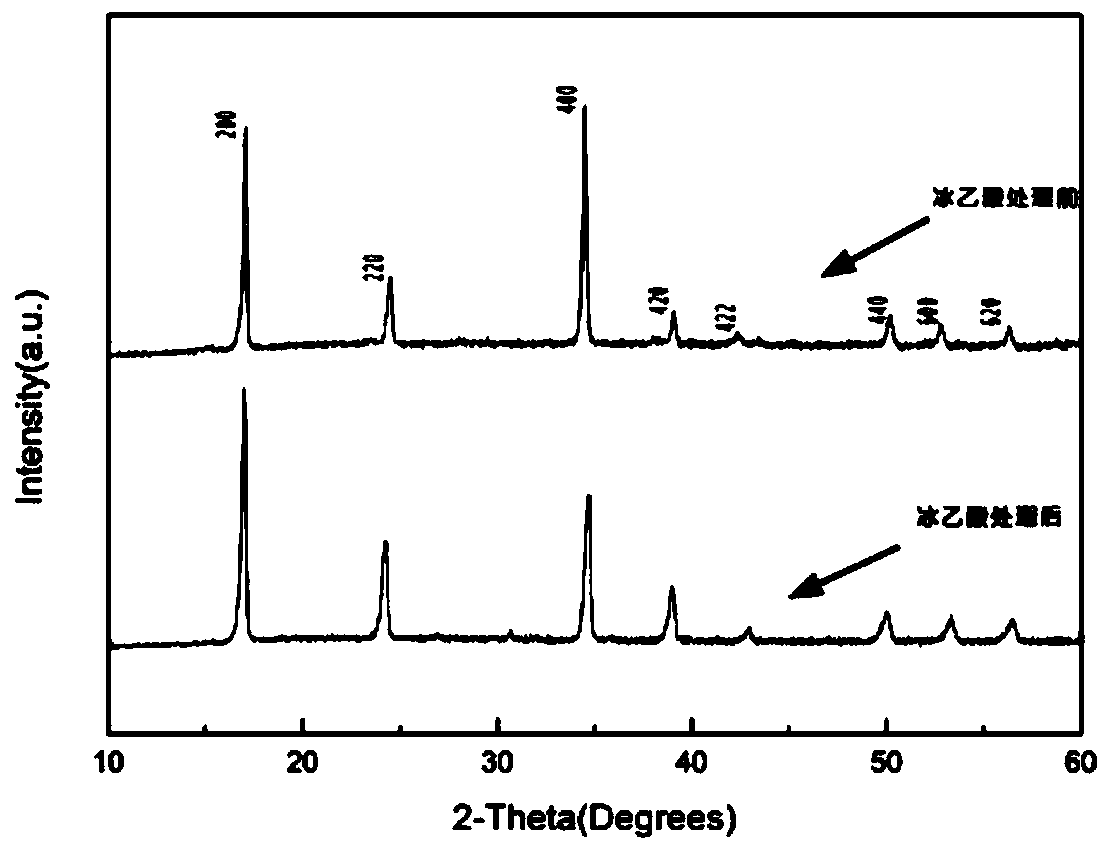
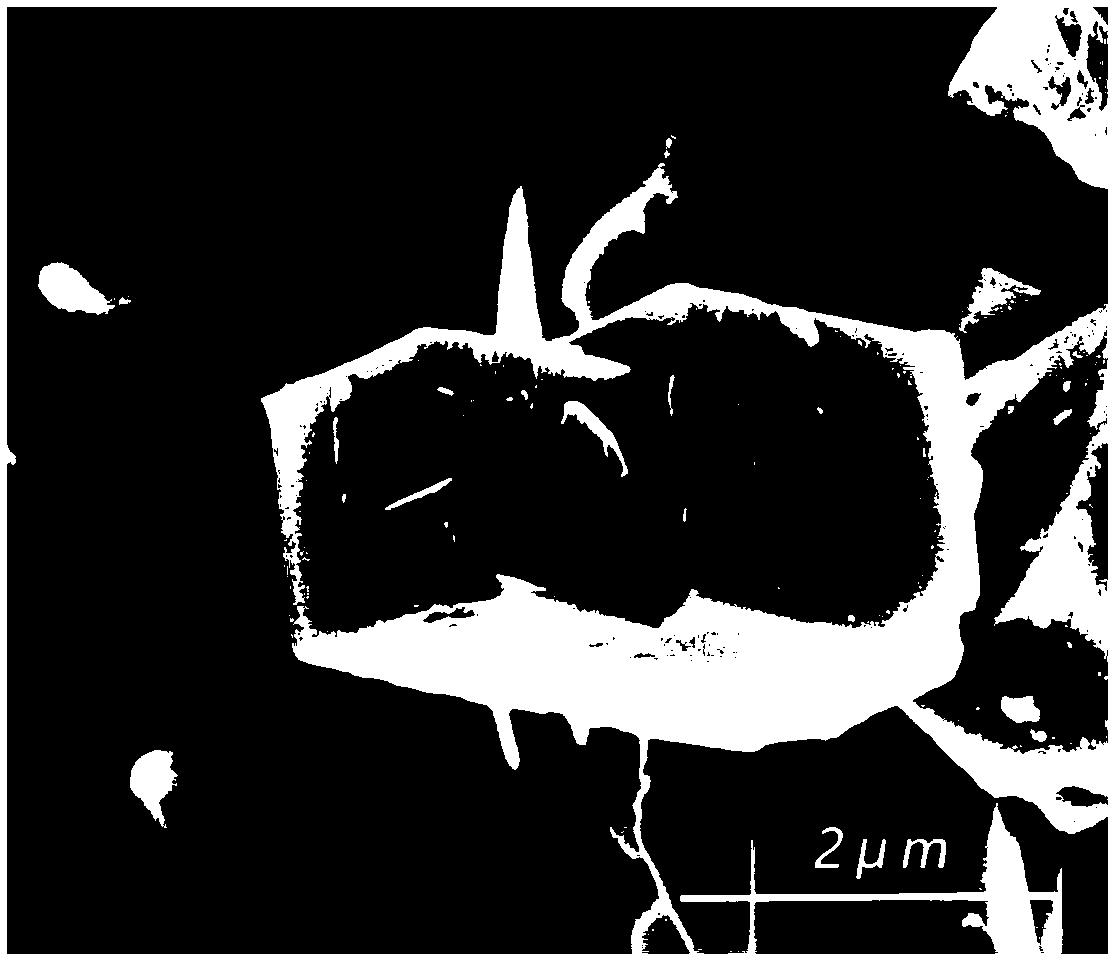
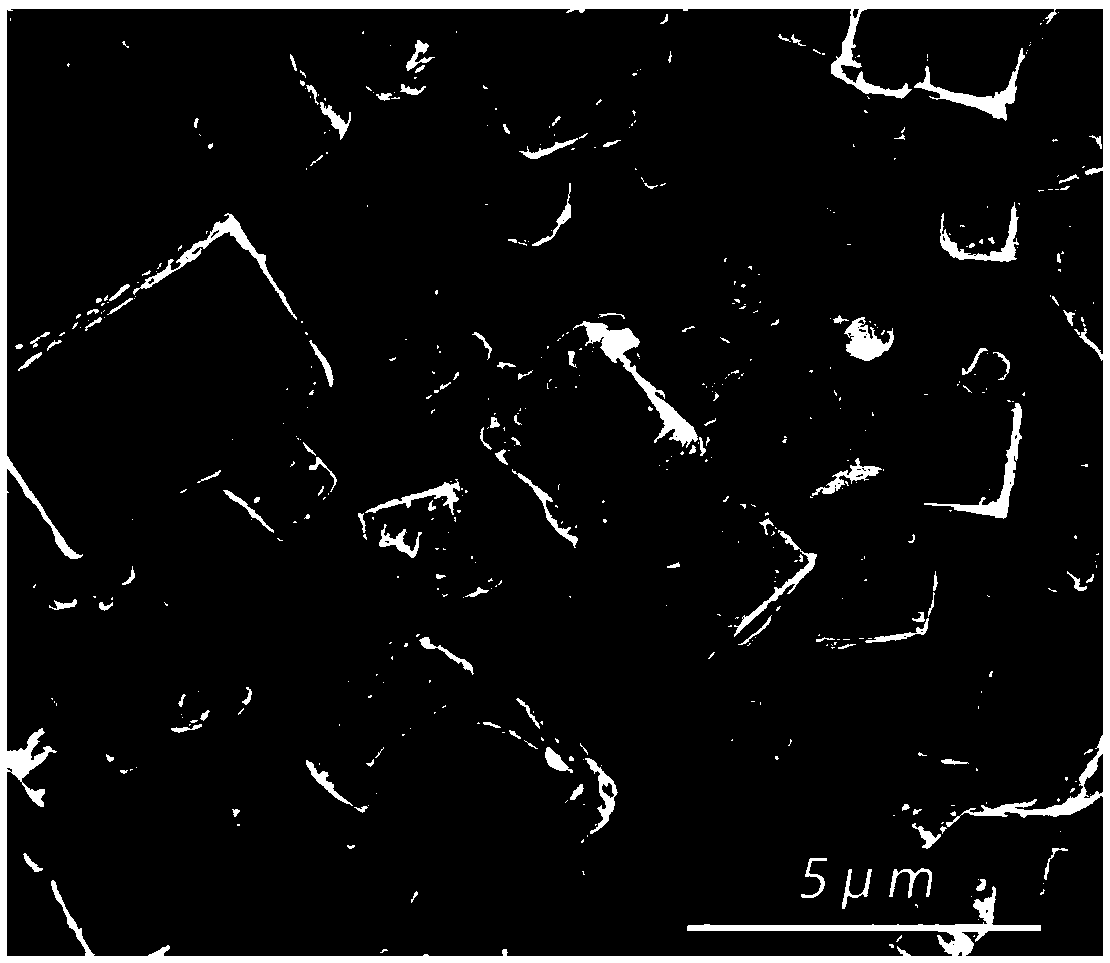
A method for modifying Prussian blue and its analogues and a sodium ion battery
A technology of Prussian blue and its analogs is applied in the modification of Prussian blue and its analogs, and the application field of sodium-ion batteries, which can solve the problems of fast decay of electrochemical performance of Prussian blue, unfavorable industrial applications, and easy water absorption again. Achieve the effects of good cycle stability, improved storage stability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the modification of Fe-PBAs by glacial acetic acid
[0037] Specifically include the following steps:
[0038] S1: Take 4mmol of Na 4 Fe(CN) 6 10H 2 O was dissolved in 200ml deionized water to form solution A; take 6mmol of FeSO 4 ·7H 2 O and 15g sodium citrate were dissolved in 200ml deionized water to form solution B;
[0039] S2: Then pour solution A into solution B, and let the mixed solution stand at room temperature (25° C.) for 6 hours;
[0040] S3: Pour off the upper clear liquid after the reaction is completed, and centrifuge and wash the obtained white precipitate three times with deionized water and absolute ethanol;
[0041] S4: Dry in a blast oven at 80°C for 12 hours to obtain aqueous Na 1.54 Fe[Fe(CN) 6 ] 0.96 (Fe-PBAs);
[0042] S5: dehydrating it at 0.1mTorr and 100°C for 30 hours to obtain dehydrated Fe-PBAs;
[0043] S6: Soak the dehydrated Fe-PBAs in anhydrous acetic acid for 12 hours, filter and dry in vacuum at 70° C. to obt...
Embodiment 2
[0046] Embodiment 2: the modification of Mn-PBAs by isopropanol
[0047] Specifically include the following steps:
[0048] S1: Take 4mmol of Na 4 Fe(CN) 6 4H 2 O was dissolved in 100ml deionized water to form solution A; take 6mmol of MnCl 2 4H 2 O and 15g sodium citrate were dissolved in 100ml deionized water to form solution B;
[0049] S2: Then solution B was slowly added dropwise to solution A, and the mixed solution was left to stand at room temperature (25° C.) for 6 hours.
[0050] S3: Pour off the upper clear liquid after the reaction is completed, and centrifuge and wash the obtained precipitate three times with deionized water and absolute ethanol respectively.
[0051] S4: Dry in a blast oven at 80°C for 12 hours to obtain aqueous Na x Mn[Fe(CN) 6 ]·zH 2 O(Mn-PBAs).
[0052] S5: dehydrating it at 100° C. for 30 hours in a vacuum of 0.1 mTorr to obtain dehydrated Mn-PBAs.
[0053] S6: Soak the dehydrated Mn-PBAs in anhydrous isopropanol for 12 hours, filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com