Application of microRNA-320(miR-320A) and its antisense nucleotide in diagnosis, prevention and treatment of cardiovascular diseases
A technology for cardiovascular and cerebrovascular diseases and coronary heart disease, which is applied in the fields of cardiovascular system diseases, gene therapy, and determination/examination of microorganisms.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
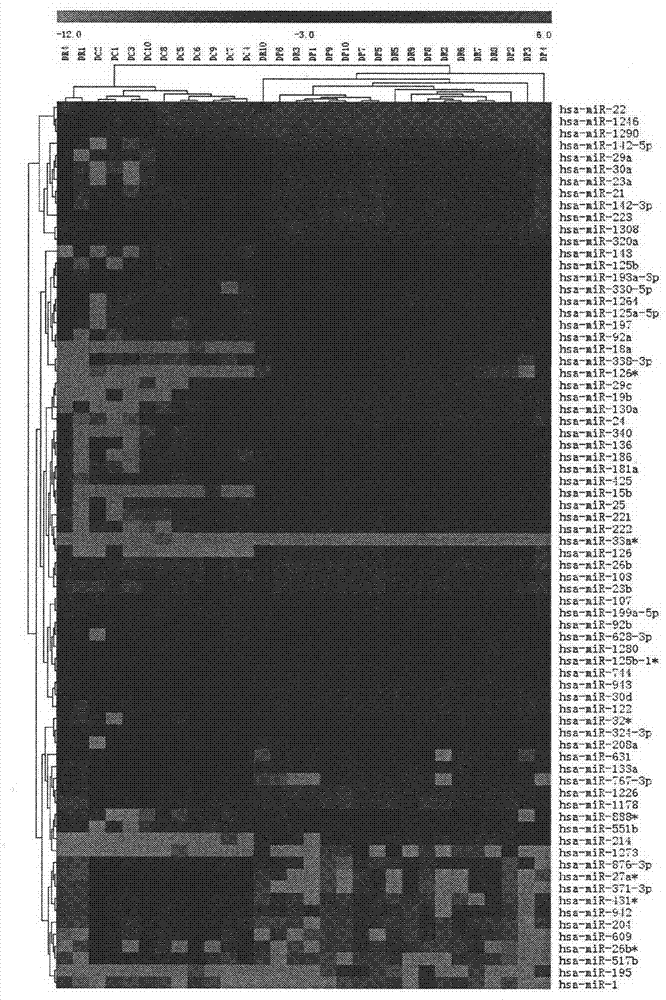
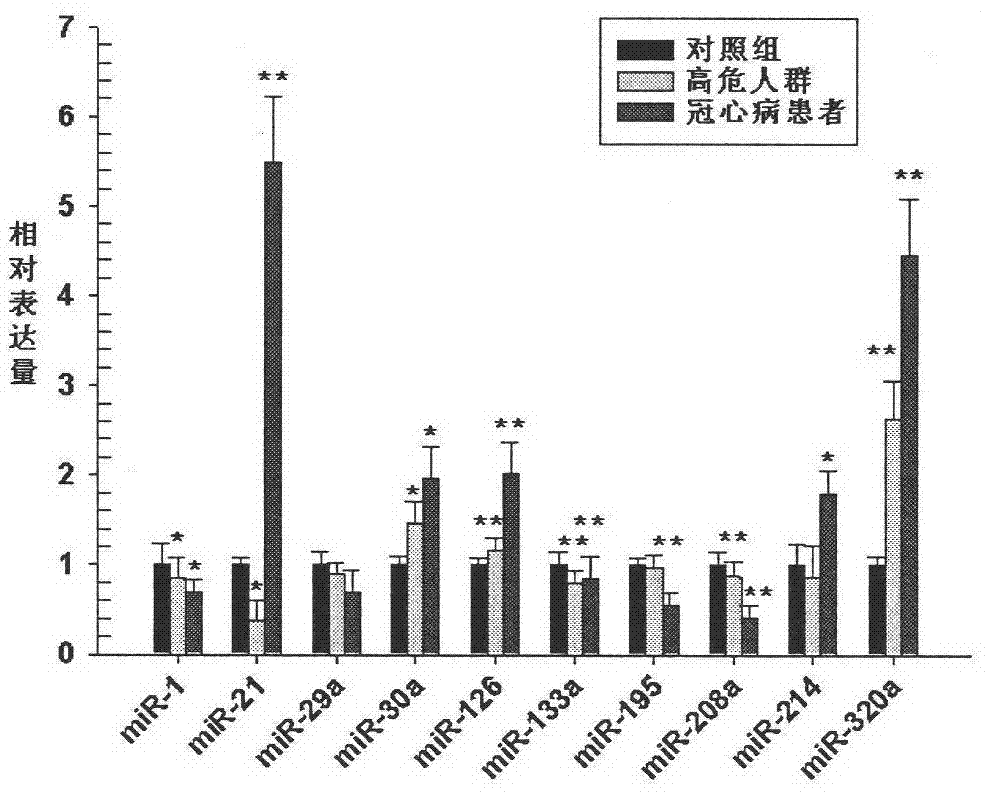
[0019] Example 1. Screening of peripheral blood miRNA expression in patients with atherosclerosis
[0020]1. Collect peripheral blood samples from 10 cases of atherosclerosis, 10 cases of acute myocardial infarction and 10 cases of normal controls (taken from patients hospitalized in the Department of Cardiovascular Medicine from 2005 to 2010, each case was confirmed by two or more clinicians Classification). After sampling, centrifuge at 3,500 rpm for 6 minutes at room temperature, take the upper layer of plasma, and store it in a -80°C refrigerator. Add 1ml TRIZOL LS (Invitrogen Company) to every 0.25ml of peripheral blood plasma, and homogenize with an electric homogenizer. After standing at room temperature for 5 minutes, add 0.2ml of chloroform, mix well, leave at room temperature for 5 minutes, and centrifuge at 12,000g at 4°C for 15 minutes. Transfer the upper aqueous phase to a new EP tube, add 0.5ml of isopropanol, mix well, place at room temperature for 10min, and ...
Embodiment 2
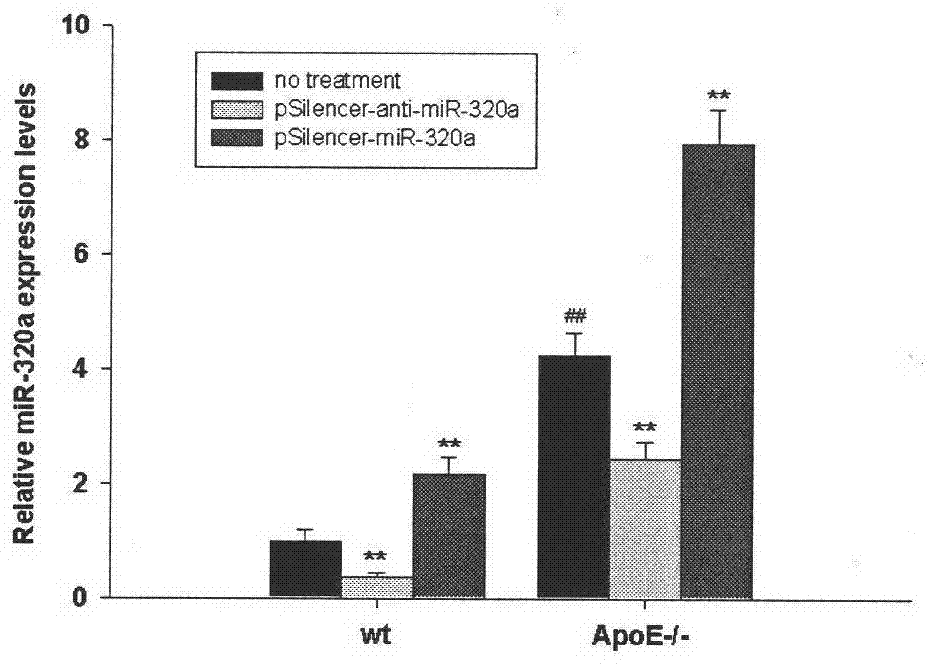
[0048] Example 2. Detection of aortic miRNA-320a expression in atherosclerotic mice
[0049] 1. In this experiment, 4-week-old ApoE - / - Mice and control mice (C57BL6) were fed with a high-fat diet for 8 weeks, and were given tail vein injections of pSilencer-miR-320a plasmid and pSilencer-anti-miR-320a plasmid at a dose of 5 mg / kg body weight. Specifically divided into 6 groups: wild-type mice control (high-fat feeding C57BL6 mice), wild-type mice miR-320a treatment intervention (high-fat feeding C57BL6+pSilencer-miR-320a), wild-type mice anti-miR- 320a treatment intervention (high fat feeding C57BL6+pSilencer-anti-miR-320a), ApoE - / - Mice (high-fat feeding ApoE - / - mice), ApoE - / - Mice miR-320a treatment intervention (high-fat feeding ApoE - / - +pSilencer-miR-320a) and ApoE - / - Mice anti-miR-320a treatment intervention (high-fat feeding ApoE - / - +pSilencer-anti-miR-320a).
[0050] 2. At the end of the experiment (week), the mouse aortic RNA was routinely extracted:
[...
Embodiment 3
[0083] Example 3. The role of miR-320a and anti-miR-320a in blood lipid regulation in atherosclerotic mice
[0084] In this experiment, 4-week-old ApoE - / - Mice and control mice (C57BL6) were fed with a high-fat diet for 8 weeks, and were given tail vein injections of pSilencer-miR-320a plasmid and pSilencer-anti-miR-320a plasmid at a dose of 5 mg / kg body weight. Specifically divided into 6 groups: wild-type mice control (high-fat feeding C57BL6 mice), wild-type mice miR-320a treatment intervention (high-fat feeding C57BL6+pSilencer-miR-320a), wild-type mice anti-miR- 320a treatment intervention (high fat feeding C57BL6+pSilencer-anti-miR-320a), ApoE - / - Mice (high-fat feeding ApoE - / - mice), ApoE - / - Mice miR-320a treatment intervention (high-fat feeding ApoE - / - +pSilencer-miR-320a) and ApoE - / - Mice anti-miR-320a treatment intervention (high-fat feeding ApoE - / - +pSilencer-anti-miR-320a).
[0085] At the end of the experiment, the whole blood of the mice was collecte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com