Method for preparing an intermediate of pitavastatin or of the salt thereof
A technology of pitavastatin and crystalline solids, which is applied in the field of preparation of intermediates of pitavastatin or its salts, can solve problems such as difficulty in meeting purity requirements, reduced yield, and longer process, so as to reduce preparation costs and prepare The effect of simple process and shortened reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045]The present invention provides a preparation method of a compound represented by Chemical Formula 4 in a crystalline solid form, the preparation method comprising the following steps: (a) making the compound represented by Chemical Formula 2 and the compound represented by Chemical Formula 3 in the presence of a base compound is reacted; and (b) adding C to the reaction mixture in step (a) 1 ~C 4 Alcohol, after forming precipitate, the precipitate obtained by washing with water, then drying, obtains the compound shown in chemical formula 4:
[0046]
[0047]
[0048]
[0049]
[0050]
[0051]
[0052] In the formula, R is a carboxylic acid protecting group. The carboxylic acid protecting group is C 1 ~C 5 Linear or branched alkyl or benzyl, preferably tert-butyl.
[0053] According to the preparation method of the present invention, the compound represented by Chemical Formula 4 can be obtained in the form of a crystalline solid, thus avoiding the dif...
Embodiment 1
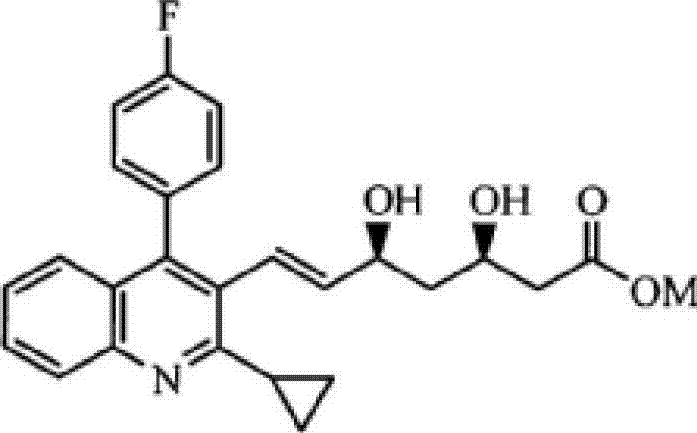
[0079](4R,6S)-(E)-6-[2-(2-cyclopropyl)-4-(4-fluorophenyl)quinolin-3-yl)-vinyl-2,2-dimethyl Preparation of -1,3-dioxane-4-yl] tert-butyl acetate (compound shown in chemical formula 4, R = tert-butyl)
[0080]
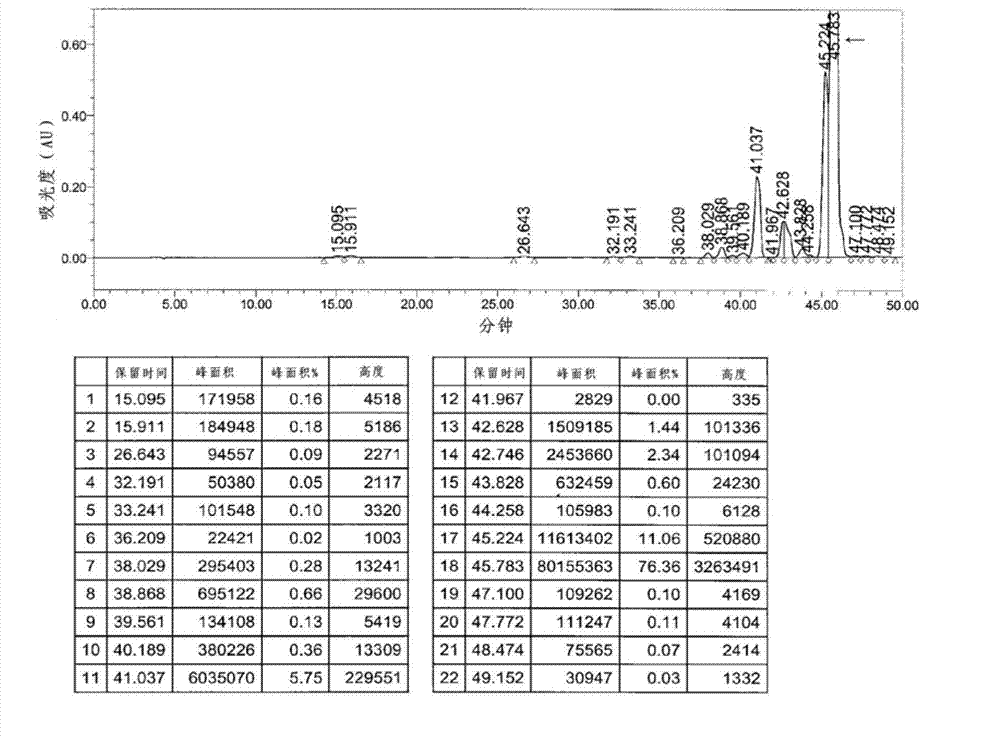
[0081] With 1.38kg of 2-((4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxane-4-yl) tert-butyl acetate (compound shown in chemical formula 3 , R=tert-butyl) was dissolved in 6 L of dimethyl sulfoxide, and 3 kg of the compound shown in Chemical Formula 2 was added, and heated to about 35° C. while stirring. After a clear solution was formed, 1 kg K 2 CO 3 , washed with 3L DMSO. The reaction mixture was heated to about 70°C and stirred for 4 hours. After confirming that there was no starting material by HPLC, the reaction was terminated. The reaction mixture was cooled to about 45°C, 18 L of methanol was added, and further cooled to about 5°C and stirred for an additional 2 hours. The resulting precipitate was separated by filtration under reduced pressure, washed with 3 L o...
Embodiment 2
[0086] (4R,6S)-(E)-6-[2-(2-cyclopropyl)-4-(4-fluorophenyl)quinolin-3-yl)-vinyl-2,2-dimethyl Preparation of -1,3-dioxane-4-yl] tert-butyl acetate (compound shown in chemical formula 4, R = tert-butyl)
[0087]
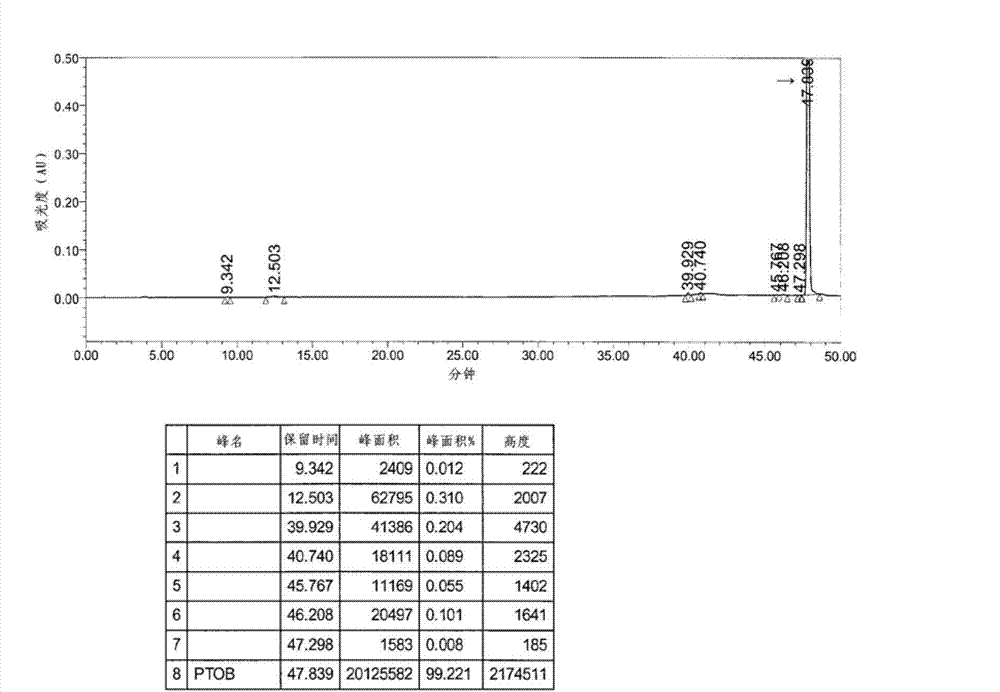
[0088] With 1.38kg of 2-((4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxane-4-yl) tert-butyl acetate (compound shown in chemical formula 3 , R=tert-butyl) was dissolved in 4 L of dimethylformamide, and 3 kg of the compound shown in Chemical Formula 2 was added, and heated to about 35° C. while stirring. After a clear solution was formed, 1 kg K 2 CO 3 , washed with 1.5 L dimethylformamide. The reaction mixture was heated to about 90°C and stirred for 2 hours. After confirming that there was no starting material by HPLC, the reaction was terminated. The reaction mixture was cooled to about 45°C, 10 L of methanol was added, and further cooled to about 5°C and stirred for an additional 2 hours. The resulting precipitate was separated by filtration under reduced pressure, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com