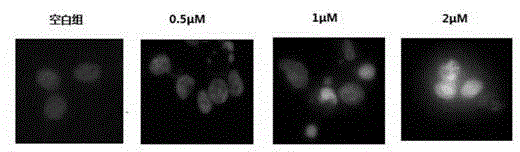
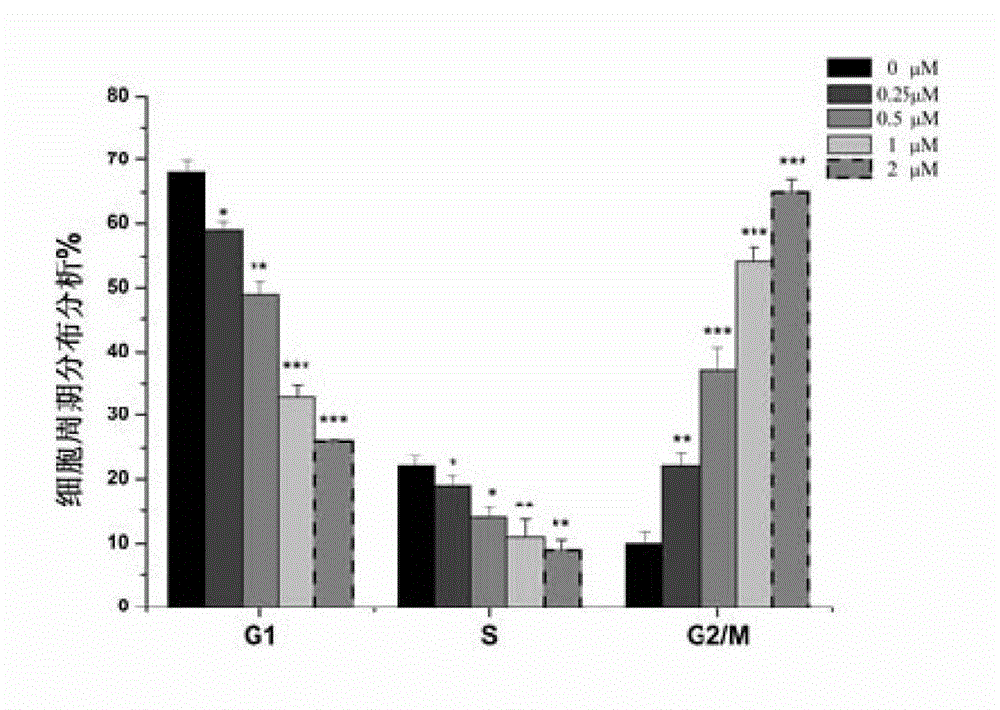
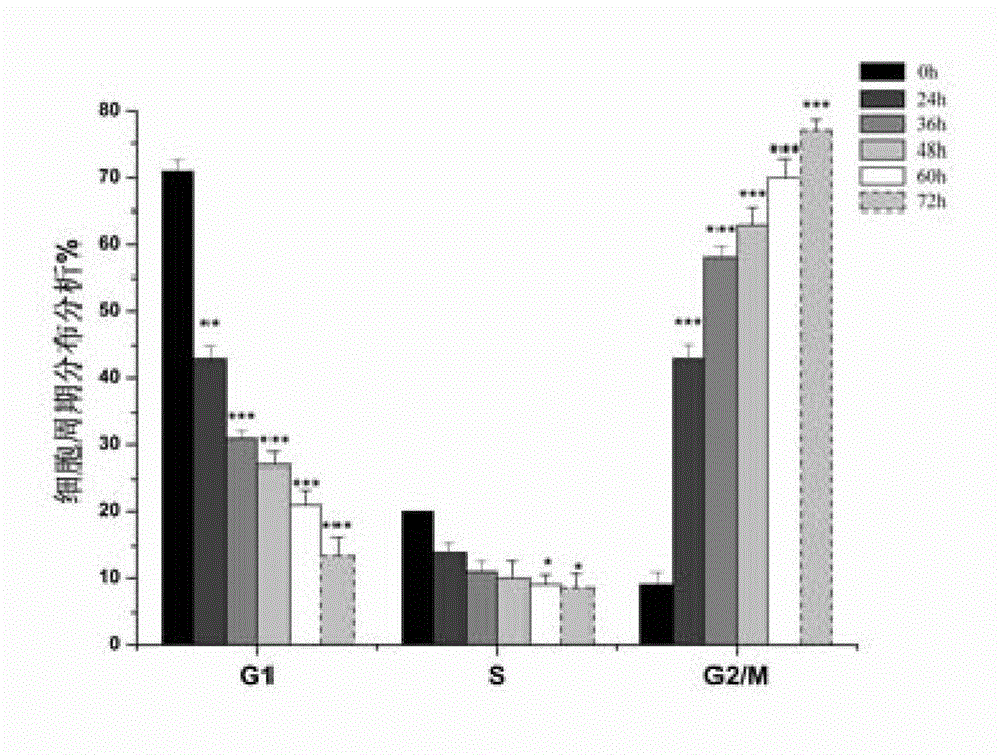
Pyrroloquinolines compound and application thereof
A kind of compound and quinoline technology, applied in the field of pyrazoloquinoline compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The method for preparing compounds shown in formulas I and II provided by the present invention specifically comprises the following steps:
[0048] (1) In an organic solvent, react 5-bromoindole with oxalyl chloride at -10°C~50°C for 2-8h, and then react with absolute ethanol at -10°C~50°C for 2-8h Obtain ethyl 2-(5-bromo-1H-indol-3-yl)-2-oxoacetate;
[0049] Among them, the molar ratio of 5-bromoindole, oxalyl chloride and ethanol is 1: (0.8~2): (2~5);
[0050] (2) In the presence of an organic solvent and an acid, mix ethyl 2-(5-bromo-1H-indol-3-yl)-2-oxoacetate with R 1 NHNH 2 HCl reacted at 80~120°C for 4~36h; or firstly mixed ethyl 2-(5-bromo-1H-indol-3-yl)-2-oxoacetate with t-BuNHNH 2 HCl was reacted at 80~120°C for 4~36h, and then in the presence of alkali, the obtained product was mixed with different halogenated aromatic hydrocarbons (R 1 X, X is Cl, Br or I) Coupling at 80~120°C to obtain the compound shown in formula III;
[0051] Among them, ethyl 2-(5...
Embodiment 1
[0069] (E)-3-(2-Phenyl-4-(2-diethylaminoethylamine)-2H-pyrazol[3,4-c]quinolin-8-yl)-N-hydroxypropene Amide dihydrochloride (Compound I-1)
[0070] (1) Preparation of ethyl 2-(5-bromo-1H-indol-3-yl)-2-oxoacetate:
[0071] Dissolve 5-bromoindole (5.9g, 30mmol) in anhydrous ether (100mL), slowly add oxalyl chloride (4.3mL, 45mmol) in anhydrous ether solution (20mL) in an ice bath, and react at room temperature 3h, after the reaction was complete, absolute ethanol (10.5mL, 180mmol) was added and reacted at room temperature for 3h. After the reaction was completed, the reaction solution was filtered, the filter cake was washed with ethanol and petroleum ether, and dried to obtain 8.3 g of a bright yellow solid (the title compound), with a yield of 93.0%.
[0072] 1 H NMR(400MHz,DMSO)δ12.55(s,1H),8.49(d,J=2.1Hz,1H),8.29(s,1H),7.54(d,J=8.6Hz,1H),7.44(dd ,J=8.6,2.1Hz,1H),4.37(q,J=7.1Hz,2H),1.34(t,J=7.1Hz,3H).
[0073] (2) Preparation of 2-phenyl-8-bromo-2H-pyrazol[3,4-c]quinolin-...
Embodiment 2
[0092] (E)-3-(2-Benzyl-4-(2-diethylaminoethylamine)-2H-pyrazol[3,4-c]quinolin-8-yl)-N-hydroxypropene Preparation of Amide Dihydrochloride (Compound I-2)
[0093](1) Preparation of 2-benzyl-8-bromo-2H-pyrazol[3,4-c]quinolin-4(5H)-one (compound Ⅲ-2):
[0094] Referring to the preparation method of compound III-1, except that the raw material phenylhydrazine hydrochloride was replaced by benzylhydrazine hydrochloride, a light yellow solid (compound III-2) was obtained with a yield of 69.1%.
[0095] 1 H NMR(400MHz,DMSO)δ11.49(s,1H),8.84(s,1H),8.16(s,1H),7.48(d,J=8.8Hz,1H),7.41–7.32(m,5H) ,7.25(d,J=8.8Hz,1H),5.61(s,2H).
[0096] 13 C NMR (101MHz, DMSO) δ156.66, 140.19, 136.28, 134.98, 129.81, 128.71, 128.09, 128.01, 126.57, 125.92, 120.49, 117.91, 117.20, 113.88, 56.45.
[0097] (2) (E)-3-(2-Benzyl-4,5-dihydro-4-oxo-2H-pyrazol[3,4-c]quinolin-8-yl)ethyl acrylate ( Compound IV-2) Preparation:
[0098] Referring to the preparation method of compound IV-1, compound III-1 was re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com