Method for membrane electrolysis of mineralized CO2 co-produced strong acid
A technology of membrane electrolysis and strong acid, applied in electrolysis process, electrolysis components, etc., can solve the problems of high energy consumption and long process flow, and achieve the effect of low energy consumption and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
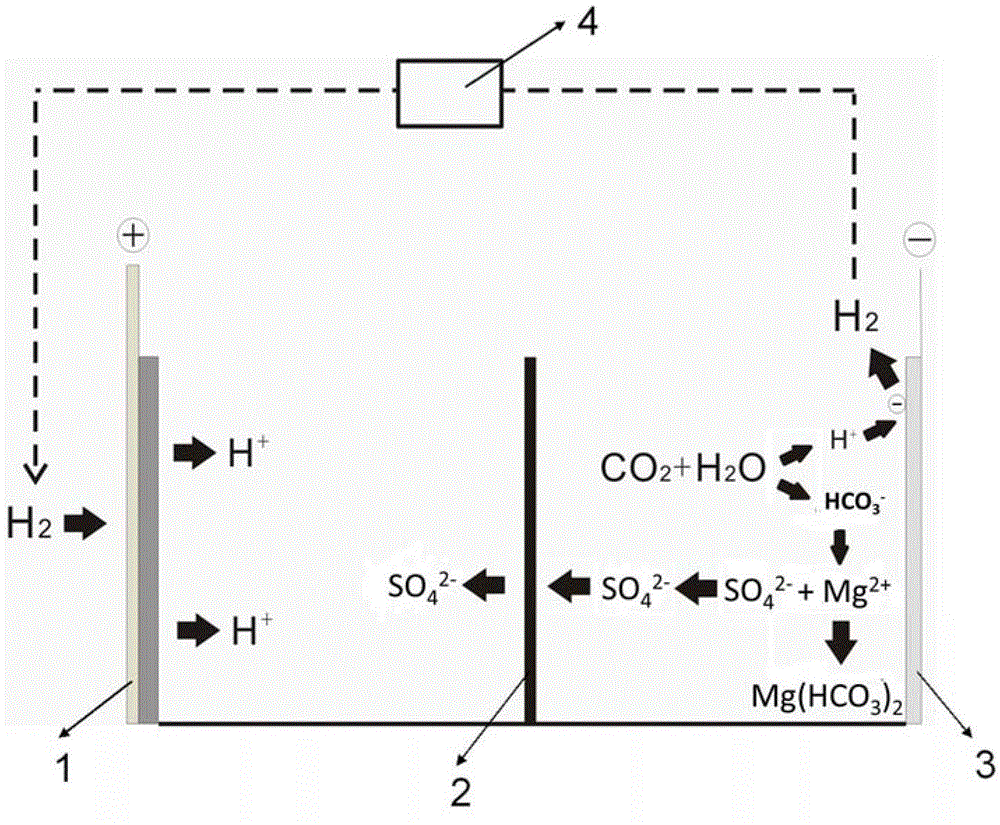
[0020] The mineralization process of the present embodiment is as attached figure 1 shown. The electrolytic cell is divided into positive and negative areas by an anion exchange membrane 2 that only allows anion to pass through but can prevent cation from passing through. Add 0.2 mol / L sulfuric acid solution to the electrolytic cell in the positive electrode area as the positive electrode electrolyte, and add 0.5 mol / L sodium sulfate solution to the electrolytic cell in the negative electrode area as the negative electrode electrolyte. The gas diffusion electrode 1 is used as the positive electrode, and the platinum electrode 3 is used as the negative electrode. Add 10g of magnesium sulfate heptahydrate into the negative electrode electrolyte, and the CO bubbled into the bottom of the electrolytic cell in the negative electrode area 2 The flow rate is 20 ml / min, the hydrogen gas generated by the negative electrode is collected and enters the buffer tank 4, and the hydrogen g...
Embodiment 2
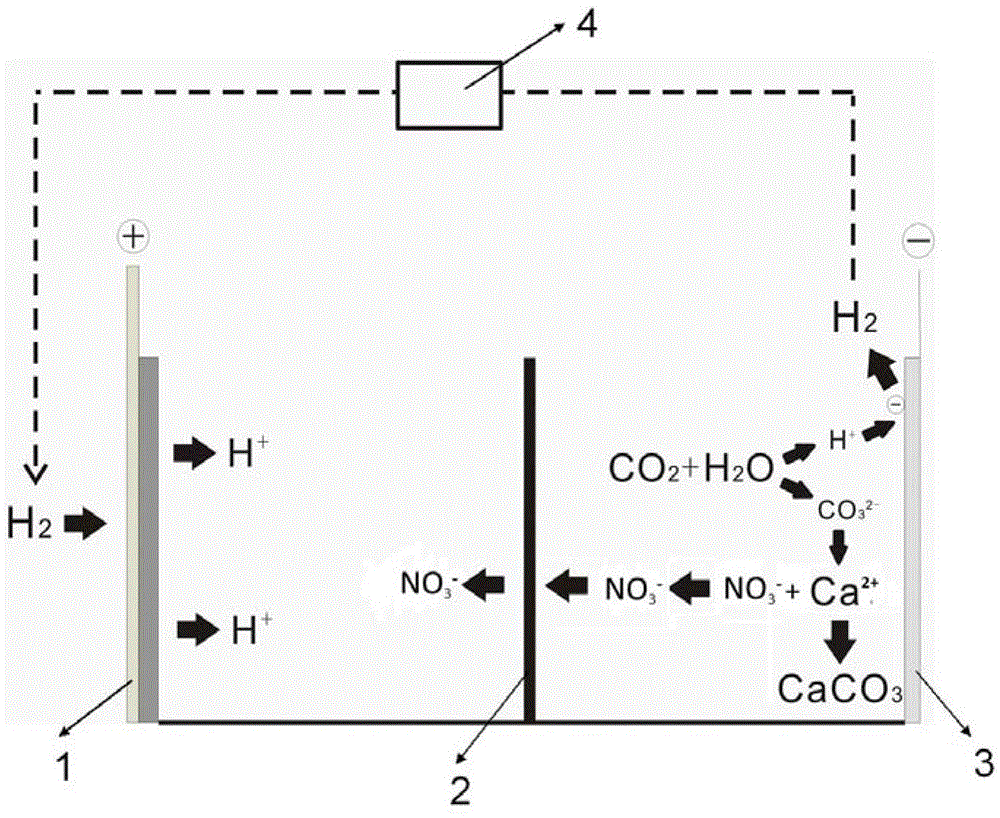
[0022] The mineralization process of the present embodiment is as attached figure 2 shown. The electrolytic cell is divided into positive and negative areas by an anion exchange membrane 2 that only allows anion to pass through but can prevent cation from passing through. Add 0.1 mol / L nitric acid solution to the electrolytic cell in the positive electrode area as the positive electrode electrolyte, and add 0.7 mol / L sodium nitrate solution to the electrolytic cell in the negative electrode area as the negative electrode electrolyte. The gas diffusion electrode 1 is used as the positive electrode, and the platinum electrode 3 is used as the negative electrode. Weigh 5g of calcium nitrate and add it to the electrolyte in the negative electrode area, and the CO bubbled into the bottom of the electrolytic cell in the negative electrode area 2The flow rate is 20 ml / min, the hydrogen generated by the negative electrode is collected and entered into the buffer tank 4, the hydroge...
Embodiment 3
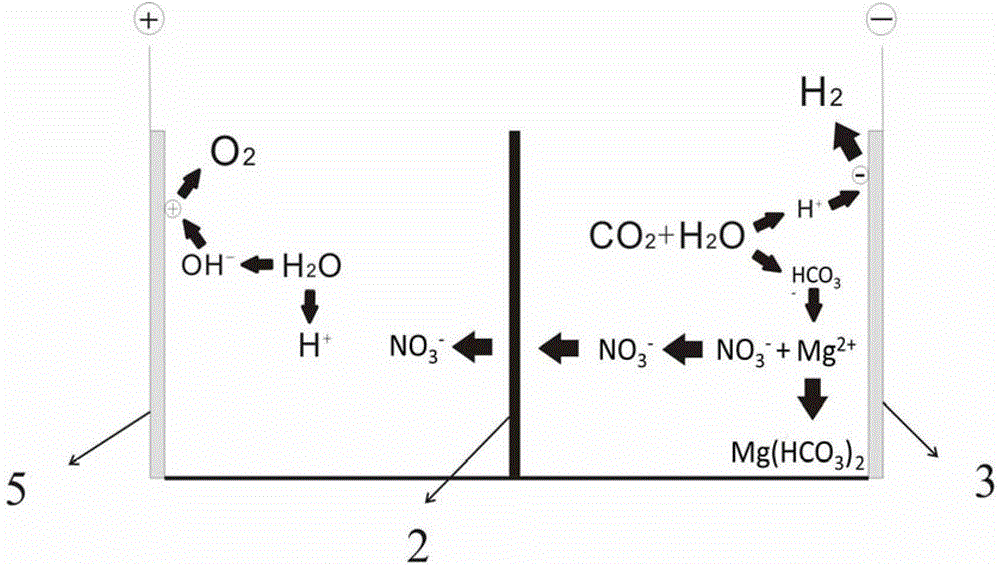
[0024] The mineralization process of the present embodiment is as attached image 3 shown. The electrolytic cell is divided into positive and negative areas by an anion exchange membrane 2 that only allows anion to pass through but can prevent cation from passing through. Add 0.1 mol / L magnesium nitrate solution to the electrolytic cell in the positive electrode area as the positive electrode electrolyte, and add 0.05 mol / L solution to the electrolytic cell in the negative electrode area as the negative electrode electrolyte. A metal platinum electrode 5 is used as the positive electrode, and a metal nickel electrode 3 is used as the negative electrode. Weigh 5g of magnesium nitrate and add it to the negative electrode electrolyte, and the CO bubbled into the bottom of the electrolytic cell in the negative electrode area 2 The flow rate was 20 ml / min, and the electrolysis reaction was carried out at a voltage of 4.1 V for 1 h. The negative electrode solution was heated at 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com