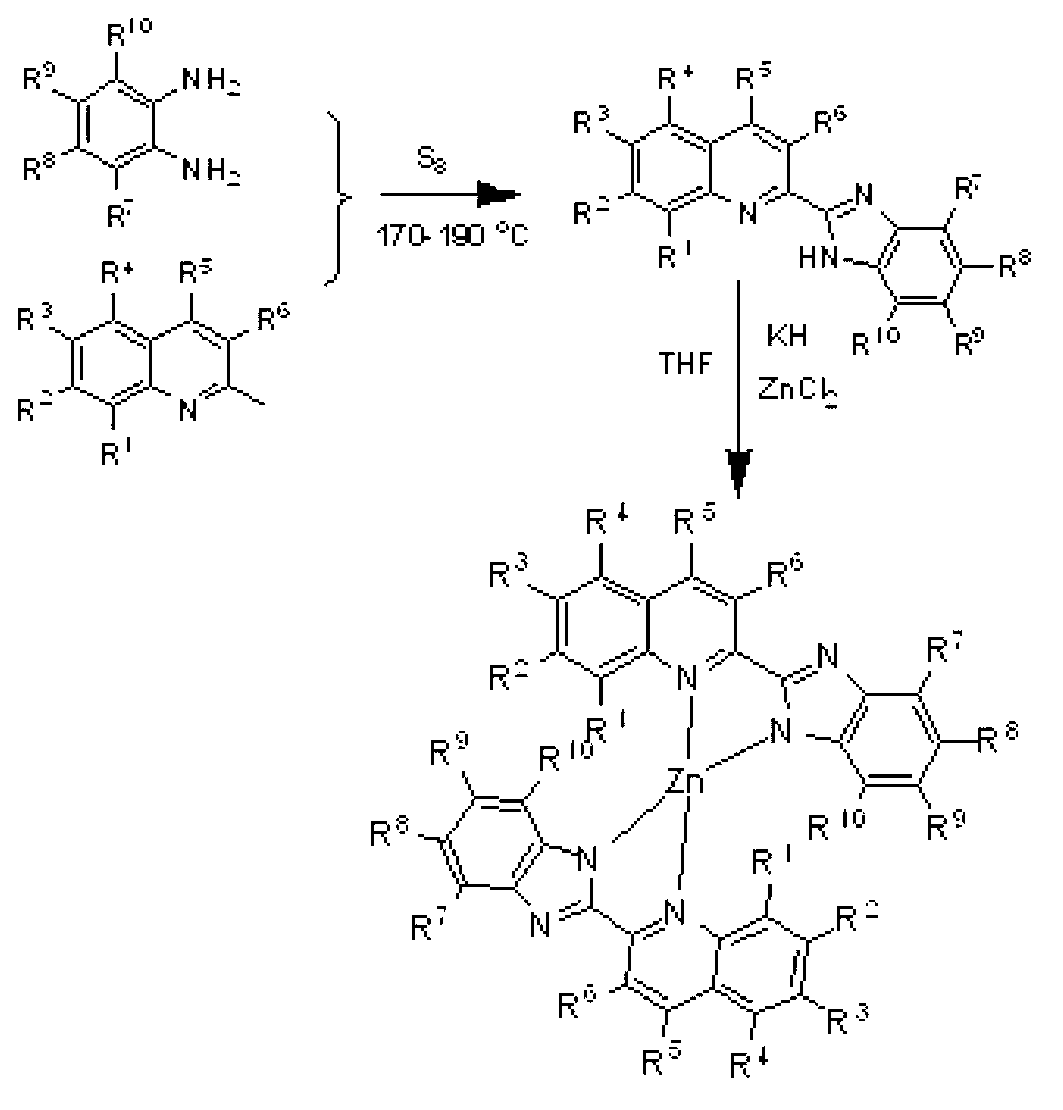
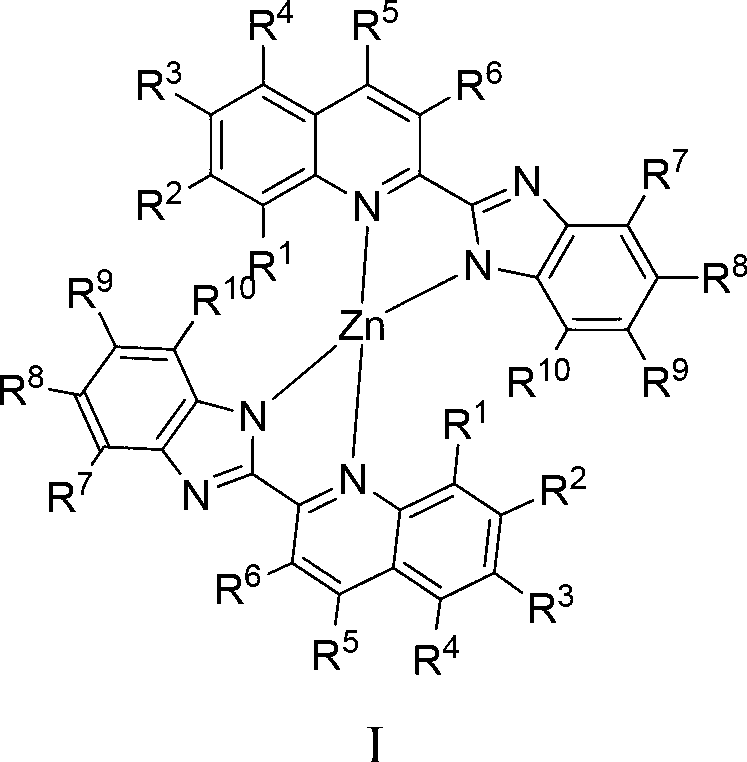
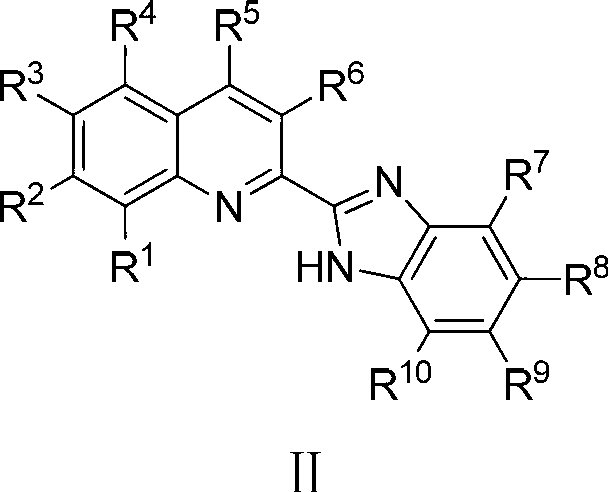
Zinc complex, preparation method thereof and application thereof in preparing anti-forgery ink
A compound and selected technology, applied in the direction of zinc organic compounds, inks, applications, etc., can solve the problems of toxic fluorescent agents or binders, low fluorescent color purity, slow drying and other problems, and achieve good fluorescent effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1, preparation of 2-benzimidazole quinoline ligand (L1) shown in formula II
[0036] Weigh 2-methylquinoline (1.43g, 10mmol), o-phenylenediamine (1.08g, 10mmol) and precipitated sulfur (7.68g, 30mmol) at 170-190°C for 12 hours, stop the reaction, and Add 30ml of methanol to terminate the reaction, cool down to room temperature, collect the concentrated reaction solution, load it on silica gel, distill off the solvent under reduced pressure, pass through a silica gel column, and rinse with eluent, which consists of petroleum ether and ethyl acetate. The volume of petroleum ether and ethyl acetate is 10:1, the third component is collected and concentrated to obtain a yellow solid powder L1 with a yield of 46%.δ H (400MHz; CDCl 3 ;Me 4 Si)10.96(1H,s),8.56(1H,d,J=8.1Hz),8.32(1H,d,J=8.6Hz),8.11(1H,d,J=8.5Hz),7.89(2H,t ,J=11.3Hz),7.75(1H,t,J=10.2Hz),7.58(1H,t,J=10.0Hz),7.49(1H,d,J=7.8Hz),7.29-7.33(2H,m ),4.46(3H,s,-CH 3 ).δ C (100MHz; CDCl 3 ;Me 4 Si) 151.1, 1...
Embodiment 2
[0037] Example 2, preparation of 2-benzimidazole-8-methylquinoline ligand (L2) shown in formula II
[0038] Weigh 2,8-dimethylquinoline (1.57g, 10mmol), o-phenylenediamine (1.08, 10mmol) and precipitated sulfur (7.68g, 30mmol) at 170-190 ° C for 12 hours of thermal melting reaction, stop Reaction, while hot, add 30ml of methanol to terminate the reaction, cool down to room temperature, collect the concentrated reaction solution, carry on silica gel, distill off the solvent under reduced pressure, pass through the silica gel column, rinse with eluent, eluent is composed of petroleum ether and ethyl acetate ester composition, the volume of petroleum ether and ethyl acetate is 8:1, the third component is collected and concentrated to obtain yellow solid powder L2, the yield is 48%.δ H (400MHz; CDCl 3 ;Me 4 Si)10.65(1H,s),8.52(1H,d,J=8.6Hz),8.27(1H,d,J=8.6Hz),7.89(1H,d,J=8.4Hz),7.76(1H,t ,J=9.9Hz),7.59(1H,t,J=10.0Hz),7.46(1H,t,J=9.8Hz),7.32(2H,m),2.87(3H,s,-CH 3 ).δ C (100MHz...
Embodiment 3
[0039] Example 3, preparation of 2-benzimidazole-8-ethylquinoline ligand (L3) shown in formula II
[0040] Weigh 2-methyl-8-ethylquinoline (1.71g, 10mmol), o-phenylenediamine (1.08, 10mmol) and precipitated sulfur (7.68g, 30mmol) at 170-190 ° C for 12 hours of thermal melting reaction , stop the reaction, add 30ml of methanol while it is hot to terminate the reaction, cool down to room temperature, collect the concentrated reaction solution, load it on silica gel, distill off the solvent under reduced pressure, pass through a silica gel column, and rinse with eluent, which consists of petroleum ether and Composed of ethyl acetate, the volume of petroleum ether and ethyl acetate is 15:1, the third component is collected and concentrated to obtain yellow solid powder L3, the yield is 39%.δ H (400MHz; CDCl 3 ;Me 4 Si)10.51(1H,s),8.52(1H,d,J=8.5Hz),8.29(1H,d,J=8.5Hz),7.90(1H,d,J=7.4Hz),7.71(1H,d ,J=8.0Hz),7.61(2H,t,J=8.6Hz),7.51(1H,t,J=10.1Hz),7.34(2H,d,J=8.3Hz),3.39-3.45(2H,m ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com