Functional preparation for adjuvant therapy of atopic dermatitis and preparation method
A technology for atopic dermatitis and adjuvant therapy, applied in the direction of anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc. problem, to restore the skin barrier function, repair the skin barrier, and achieve the effect of good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
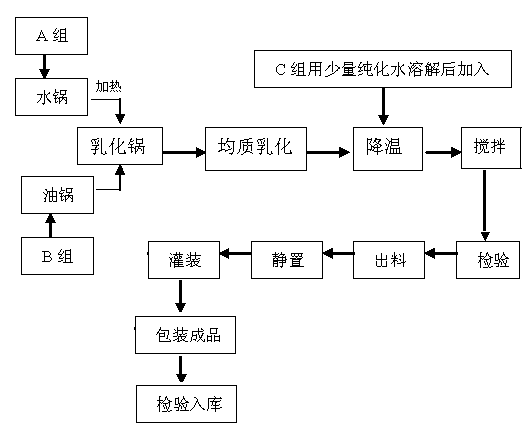
[0017] The weight components of the main raw materials of the functional preparation of the present invention are: 0.2 parts of sodium hyaluronate, 5 parts of emulsifying thickener, 5 parts of oil, plus deionized water to 100 parts.
[0018] Preparation method: Add the emulsified thickener (component A) into the water pot and heat it to 75°C; add the oil (component B) into the oil pot and heat it to 75°C; mix the solution of group A and the solution of group B Mix in an emulsifying pot and homogeneously emulsify for 10 minutes; cool the above emulsion to 40°C, add sodium hyaluronate as the main raw material, stir for 20 minutes, discharge, stand still, fill and pack.
Embodiment 2
[0020] The main raw material components of the functional preparation of the present invention are: 0.5 parts of sodium hyaluronate, 8 parts of emulsifying thickener, 8 parts of oil, and deionized water to 100 parts.
[0021] Preparation method: Add the emulsified thickener (component A) into the water pot and heat it to 78°C; add the oil (component B) into the oil pot and heat it to 78°C; mix the solution of group A and the solution of group B Mix in an emulsifying pot and homogeneously emulsify for 12 minutes; cool the above emulsion to 42°C, add sodium hyaluronate as the main raw material, stir for 23 minutes, discharge, stand still, fill and pack.
Embodiment 3
[0023] The functional preparation of the present invention has main raw material components by weight: 1 part of sodium hyaluronate, 10 parts of emulsifying thickener, 10 parts of oil, and deionized water to 100 parts.
[0024] Preparation method: Add the emulsified thickener (component A) into the water pot and heat it to 80°C; add the oil (component B) into the oil pot and heat it to 80°C; mix the solution of group A and the solution of group B Mix in an emulsifying pot and homogeneously emulsify for 15 minutes; cool the above emulsion to 45°C, add sodium hyaluronate as the main raw material, stir for 25 minutes, discharge, stand still, fill and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com