The method of styrene epoxidation reaction
A technology of epoxidation reaction and styrene, which is applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve the problems of poor catalyst stability and achieve the effects of good stability, low cost and excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 10 millimoles of o-aminophenol, put it into a round-bottomed flask (50 milliliters) containing 20 milliliters of absolute ethanol, heat and stir at 50° C., add 10 millimoles of 2-acetylpyridine after the o-aminophenol is completely dissolved, and stir for 1 After 6 days, the light yellow polyhedral crystals were separated by suction filtration and washed with a small amount of ethanol to obtain 2-acetylpyridine o-aminophenol Schiff base complex.
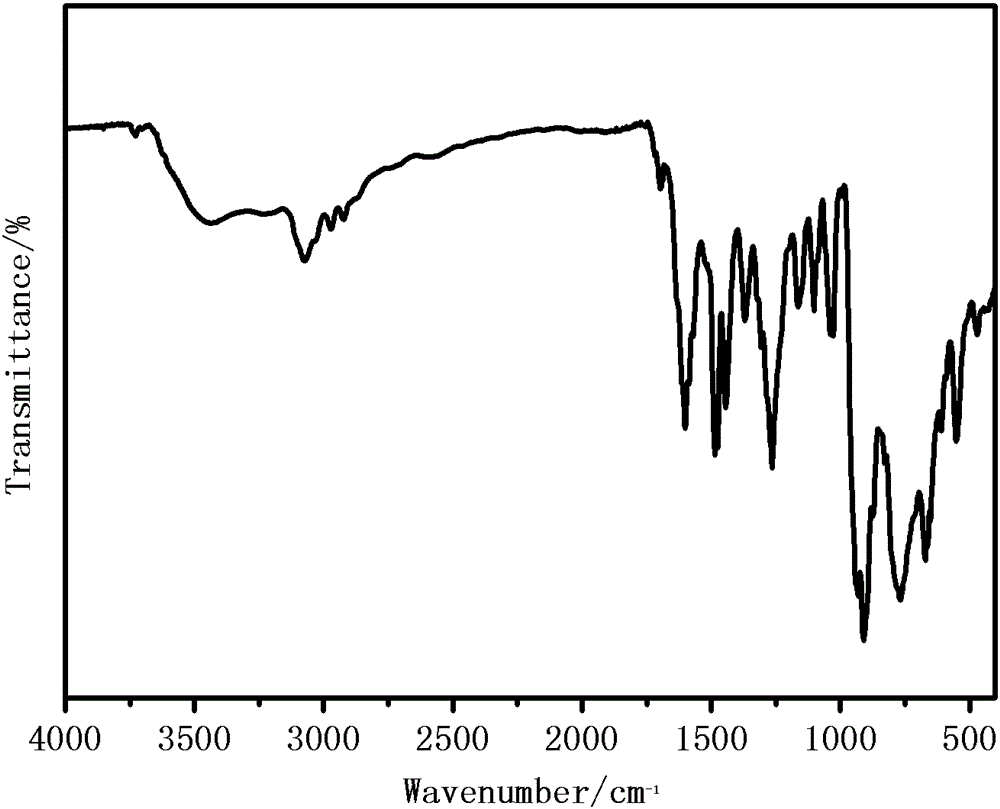
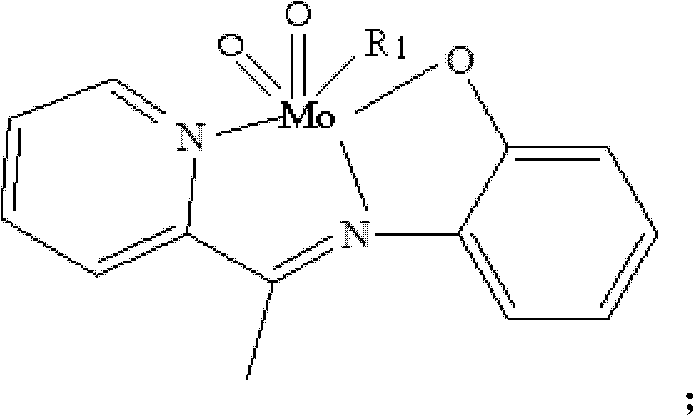
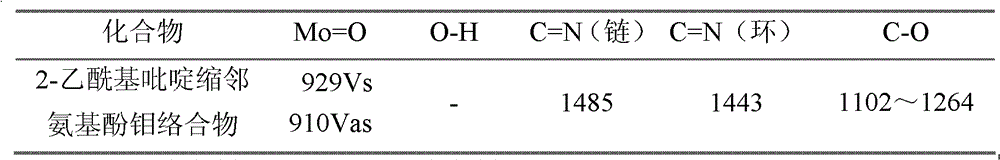
[0021] Weigh 5mmol of 2-acetylpyridine-o-aminophenol, put it into a round-bottomed flask (50ml) containing 15ml of absolute ethanol, heat and stir at 75°C, and add 5mmol of acetylacetone after the 2-acetylpyridine-o-aminophenol is completely dissolved Molybdenum, stirred brown powder to precipitate, continue to heat and stir for 2 hours, then cool and suction filter, wash with ethanol and dry in vacuum at 40°C to obtain a brown powder product, its structural formula is as follows, and its infrared analysis spectrum is sho...
Embodiment 2
[0024] Weigh 10 mmol of o-aminophenol, put it into a round-bottomed flask (50 ml) containing 20 ml of pyridine, heat and stir at 50°C, add 10 mmol of 2-acetylpyridine after the o-aminophenol is completely dissolved, and stir for 1 hour After standing for 6 days, the light yellow polyhedral crystals were separated by suction filtration and washed with a small amount of ethanol to obtain 2-acetylpyridine o-aminophenol Schiff base complex.
[0025] Weigh 5mmol of 2-acetylpyridine-o-aminophenol, put it into a round-bottomed flask (50ml) containing 15ml of pyridine, heat and stir at 75°C, and add 5mmol of molybdenum acetylacetonate after the 2-acetylpyridine-o-aminophenol is completely dissolved. Stir brown powder to precipitate, continue to heat and stir for 2 hours, then cool and suction filter, wash with pyridine and dry in vacuum at 40°C to obtain the product. Its structural formula is as follows, and its infrared analysis spectrum is consistent with figure 1 resemblance.
[0...
Embodiment 3
[0028] Take 2.5mmol (0.29ml) of styrene, 5mmol (0.72ml) of tert-butyl hydroperoxide (TBHP), 6ml of benzene as solvent, and 0.025mmol of catalyst, put them into a 25ml one-necked flask, stir in an oil bath at 80°C, condense and reflux 9h. The molar ratio of styrene to catalyst was 100:1.
[0029] Reaction result: the conversion rate of styrene is 69.35%, and the selectivity of styrene oxide is 80.19%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com