Compound for preparing pyrimidinedione DPP-IV (dipeptidyl peptidase IV) inhibitors
A DPP-IV, pyrimidinedione technology, applied in the preparation of pyrimidinedione DPP-IV inhibitors, new intermediates and the field of preparation thereof, can solve the problems of low purity and low yield of compound 4
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
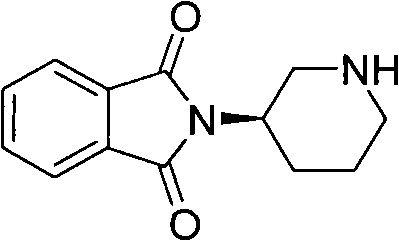
[0066] The synthesis of embodiment 1 compound I'
[0067]
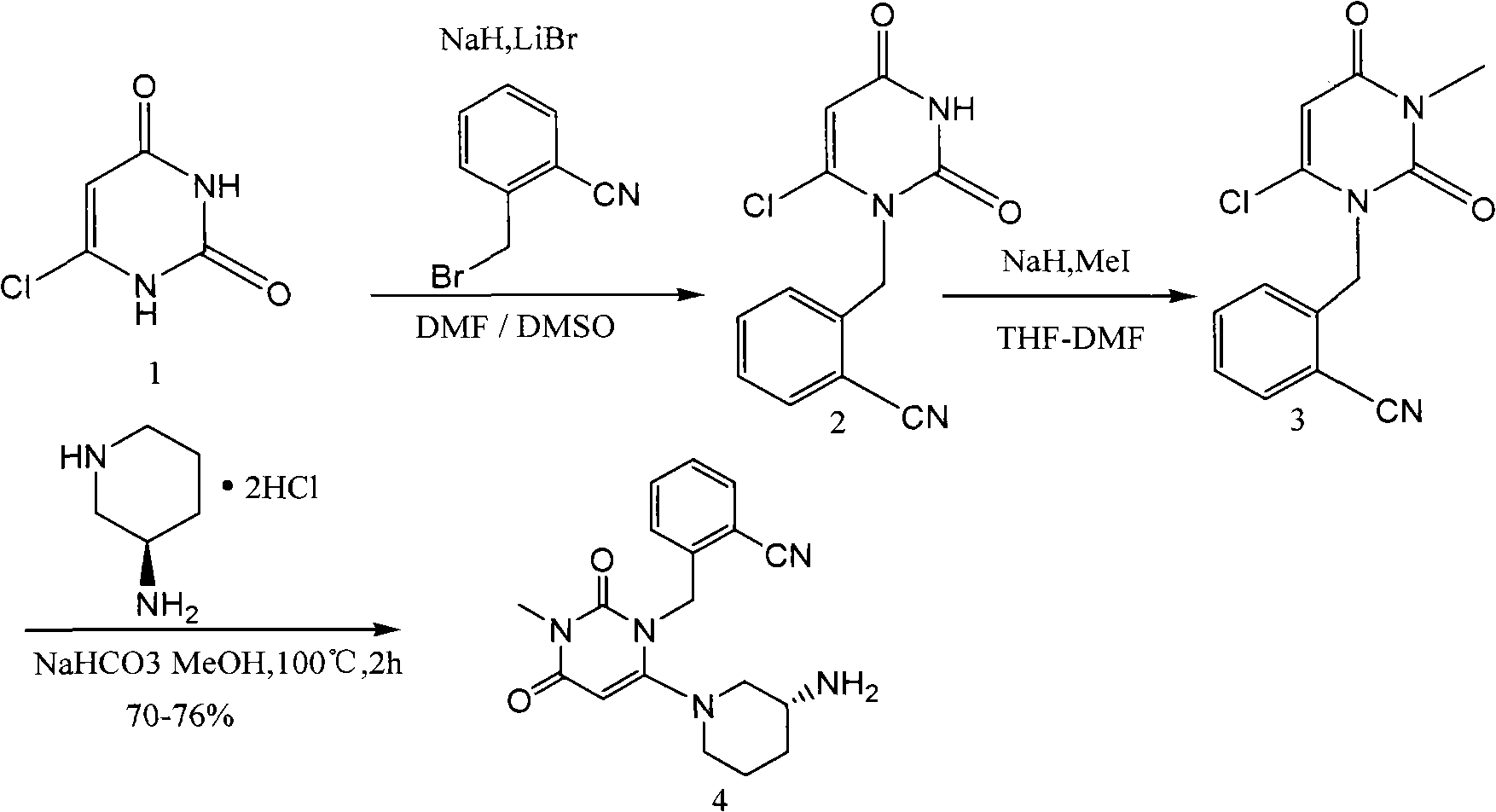
[0068] Synthesis of compound 1-1:
[0069]
[0070] In a 100mL three-necked flask, add R-3-aminopiperidine dihydrochloride (5.0g, 0.029mol), phthalic anhydride (4.3g, 0.029mol), acetic acid (15mL), under stirring, control the temperature to 130°C, traced by TLC, after the basic reaction of the raw materials is complete, cool down to 80°C, add ethanol solution of hydrogen chloride (25%, 15mL), stir for about 30min, there is solid precipitation, continue to stir for 1h, move to room temperature and stir overnight, 0°C Let it stand for 3h, filter, and wash the filter cake with absolute ethanol, and add the resulting white solid to CH 2 Cl 2 (50mL) and water (20mL) mixed solution, with Na 2 CO 3 Adjust the pH to 9-10 with a saturated aqueous solution, extract and collect the organic phase, and wash the aqueous phase with CH 2 Cl 2 (50mL×2 times) extraction, combined organic phase, anhydrous Na 2 SO 4 Dry, fi...
Embodiment 2
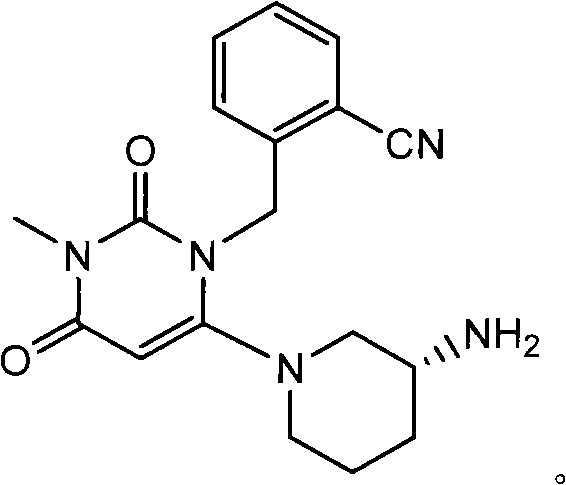
[0086] Example 2 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H) The synthesis of -yl} methyl) benzonitrile:
[0087]
[0088] Compound I' (4g, 8.5mmol) was dissolved in a mixed solvent of ethanol (16mL) and hydrazine hydrate (4mL), reacted under reflux conditions, a large amount of solids were precipitated, filtered, and dissolved in CH 2 Cl 2 (60mL) washes the filter cake, adds 40mL water to the filtrate, extracts the organic phase, and the aqueous phase is distilled with CH 2 Cl 2 Extraction (60mL × 2 times), the organic phase was combined, washed with saturated NaCl aqueous solution (150mL × 2 times), the organic phase was washed with anhydrous NaCl 2 SO 4 Dry, filter, decolorize with activated carbon, and evaporate the solvent under reduced pressure to obtain 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-diox Substituted-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile 2.75g (yield 95%).
[0089] 1 HNMR (500MHZ, CDCl 3 ): δ7.6...
Embodiment 3
[0098] The synthesis of embodiment 3 compound 2' and 2
[0099]
[0100] Compound 2' was prepared using the method for preparing compound I' described in Example 1, but using 2-bromomethyl-4-fluorobenzylcyanide instead of o-cyanobenzyl bromide, refer to 2-({6-[(3R) The preparation method of -3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile is prepared Compound 2.
[0101] Compound 2'MS m / z (ESI): 488 (M+1).
[0102] Compound 2 MS m / z (ESI): 358 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com