Novel anti-CD98 antibody and use thereof
An antibody and antibody heavy chain technology, applied in the fields of diabetes therapeutics, rheumatoid arthritis therapeutics, and autoimmune disease therapeutics, can solve the problem that anticancer drugs have not yet been developed, and achieve the goal of curbing fatigue and exhaustion, and curbing the incidence of disease. and progress, the effect of fewer side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
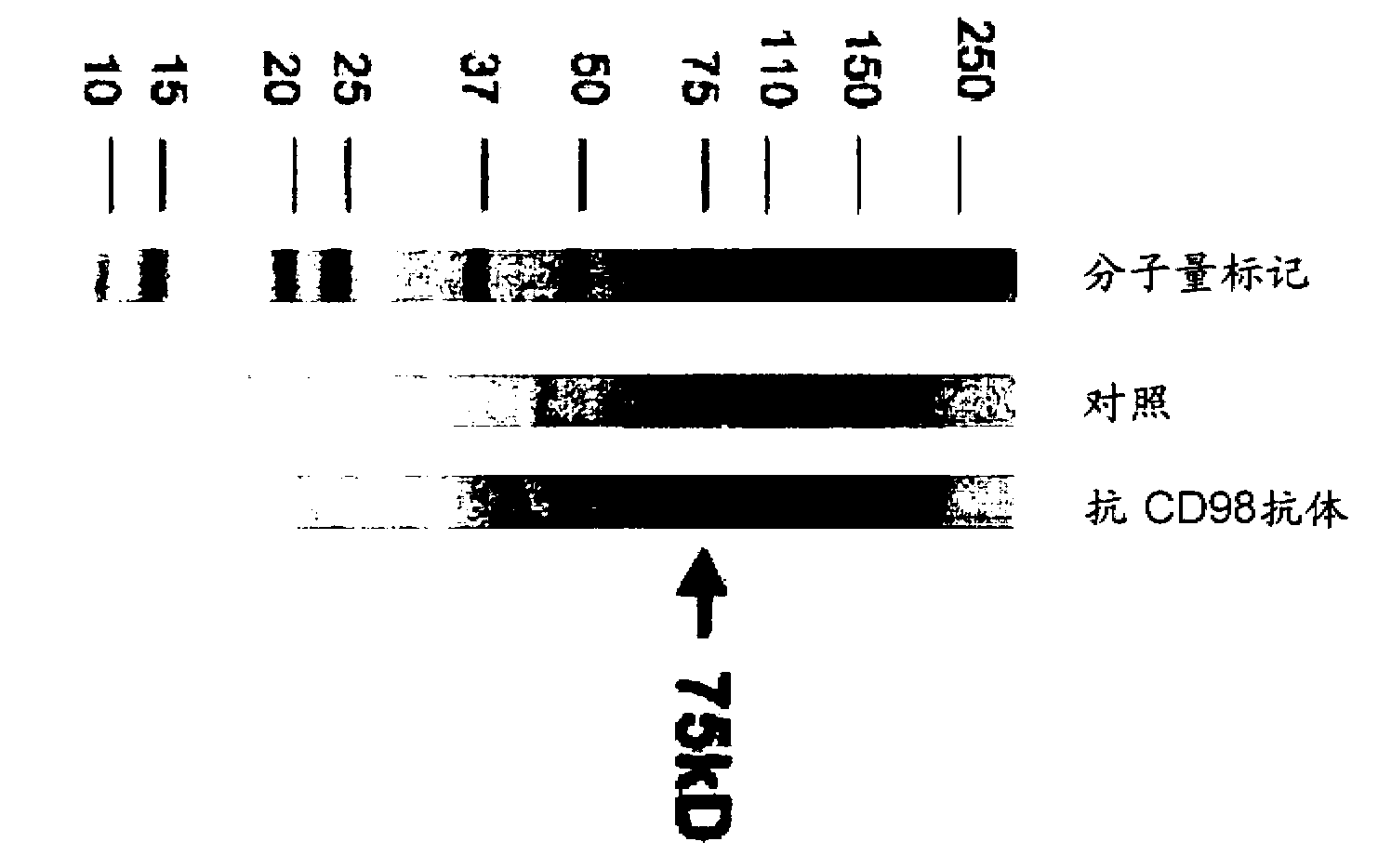
[0141] (Example 1) Preparation of anti-mouse CD98 antibody
[0142] Bone marrow cells were isolated from BALB / c mice and cultured for 8 days in the presence of GM-CSF. Then, 8-week-old Wistar rats (1×10 7 cells). Two weeks after the first immunization, the immunization was repeated. Next, 2 weeks later, the mice were immunized again, and 3 days later, the spleens were removed from the rats. Using spleen cells and SP2 cells (myeloma cells), cell fusion was performed according to a conventional method, and selection was performed in HAT medium. Then, antibodies reactive to dendritic cells were selected using flow cytometry. For selected antibodies, single clones were collected by limiting dilution. Since the limiting dilution method was performed three times at 0.5 cells / well, the clones were considered to be single clones. No less than 40 kinds of antibodies can be isolated by the above method.
[0143] The obtained antibodies were added to a T cell culture system (a mix...
Embodiment 2
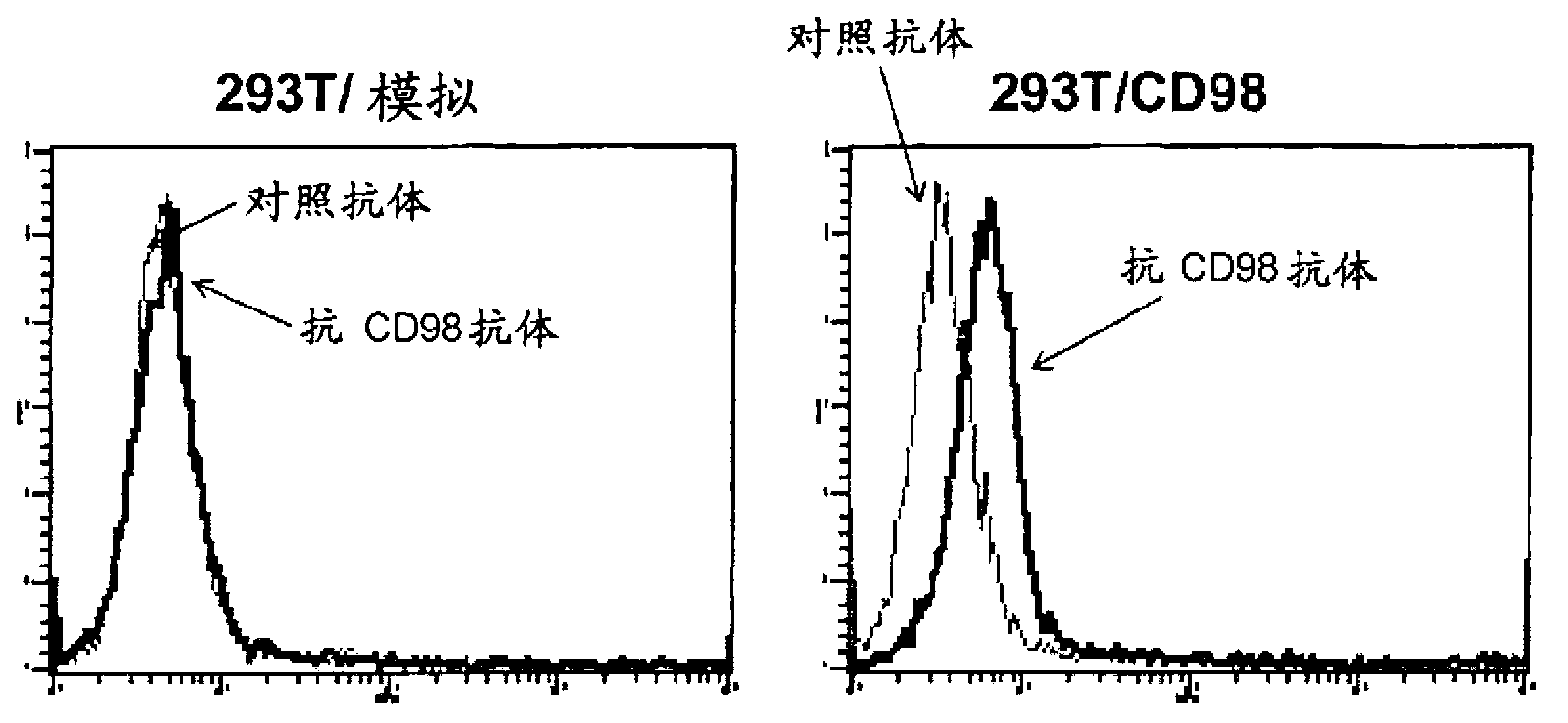
[0145] (Example 2) Reactivity of the anti-mouse CD98 antibody (mCD98Ab) of the present invention
[0146] In order to confirm the reactivity of the mCD98Ab of the present invention, CD98-expressing CHO cells (293T / CD98) were generated by introducing the mouse CD98 gene (mCD98, GenBank / EMBL / DDBJ Accession No. U25708). That is, pcDNA3.1 introduced with the mouse CD98 gene or pcDNA3.1 alone was transfected into CHO cells by electroporation. To select cells in which the CD98 gene was incorporated into the CHO cell genome, drug-resistant cells were isolated by performing drug screening using G418. Cells were stained with rat IgG (control antibody) or mCD98 Ab, washed to remove free antibody, and stained with PE-labeled anti-rat IgG. The result is as figure 2 shown. The mCD98Ab of the present invention does not react with cells without CD98 gene transfer, but reacts with cells with CD98 gene transfer. The left panel shows cells transfected with pcDNA3.1 alone, and the right pan...
Embodiment 3
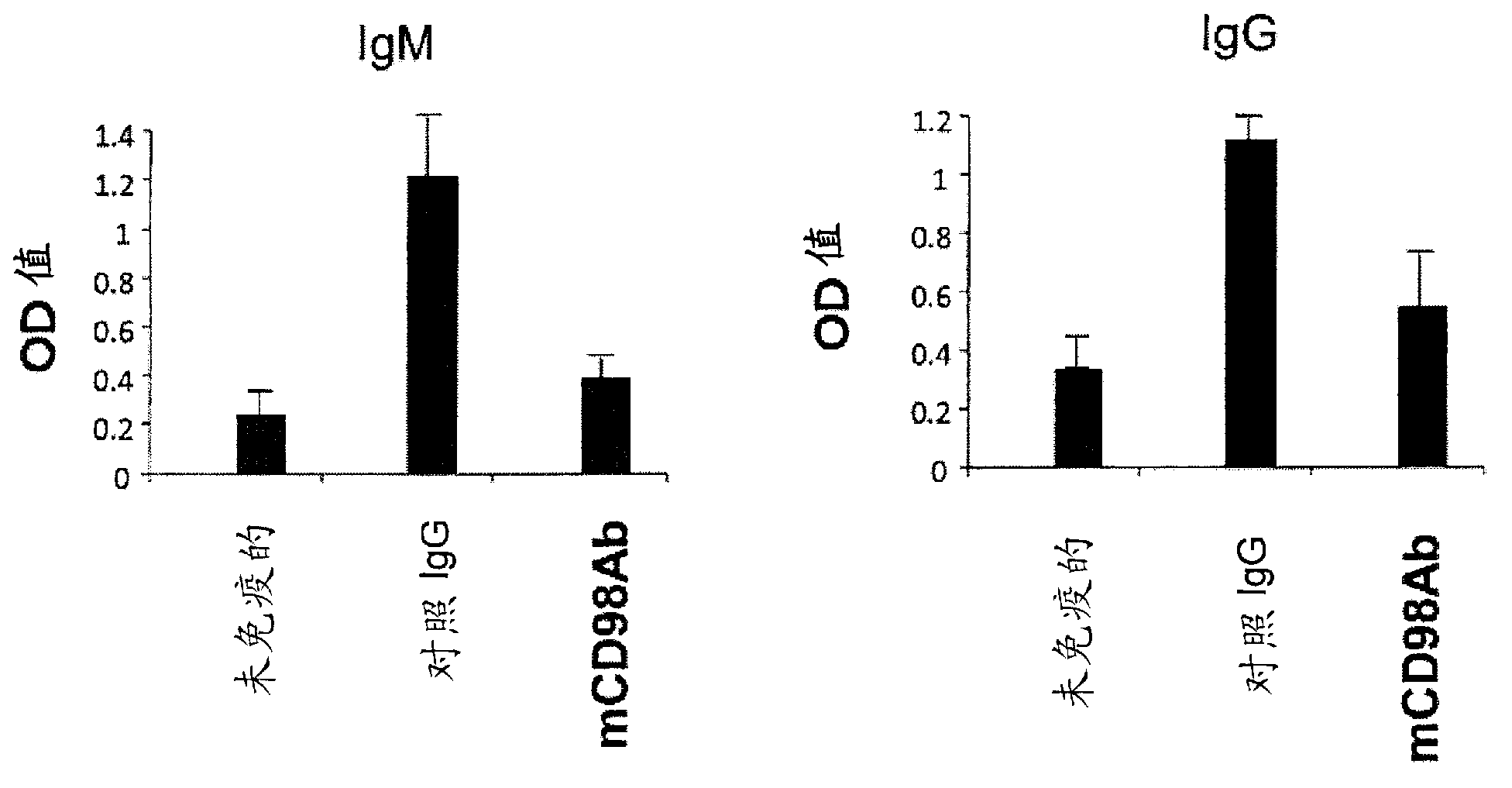
[0148] (Example 3) Anti-inflammatory effect of the anti-mouse CD98 antibody (mCD98Ab) of the present invention
[0149] In order to evaluate the function of the mCD98Ab of the present invention, an OVA immunization model was prepared. That is, BALB / c mice were immunized and sensitized by subcutaneously injecting a complex of OVA (ovalbumin) protein mixed with complete Freund's adjuvant. Taking the first day of immunization and sensitization of mice as day 0, control antibody or anti-CD98 antibody (each 200 μg) was intraperitoneally administered to mice on day 2, 4, 6 and 8. On day 10, sera were isolated from mice, and OVA-specific IgM and IgG therein were measured by ELISA method. That is, ovalbumin was immobilized on a 96-well plate at a concentration of 50 µg / ml, and reacted with the collected serum. Anti-ovalbumin antibodies were detected with HRP-labeled anti-mouse IgG.
[0150] The result is as image 3 shown. exist image 3 Among them, a significant reduction in OV...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com