Preparation method of fish iridovirus DNA (Deoxyribonucleic Acid) vaccine
A DNA vaccine and iridescent virus technology, applied in the field of biomedicine, can solve the problems of restricting the promotion of vaccines and high production costs of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the preparation of DNA vaccine
[0019] Vector build:
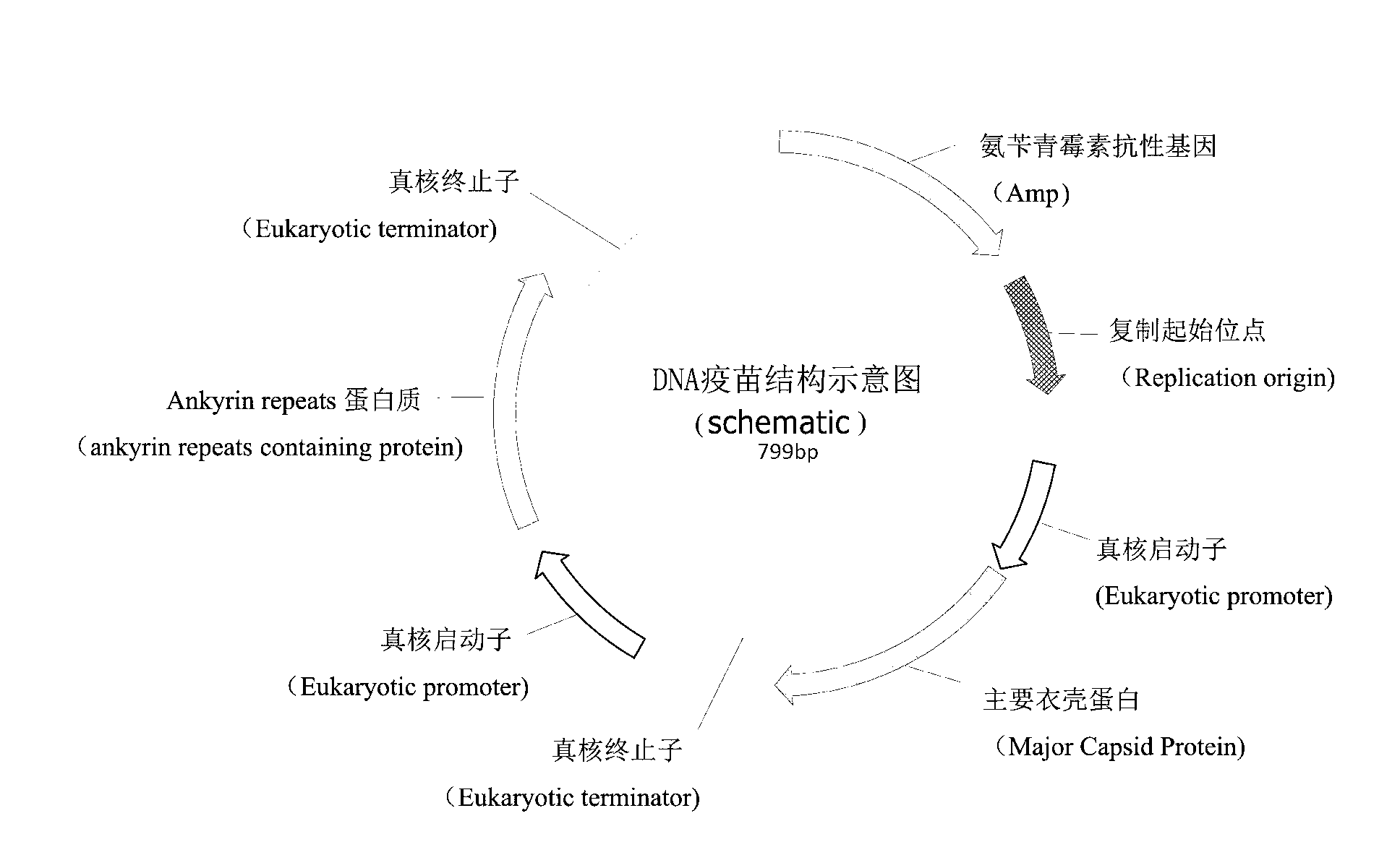
[0020] The nucleotide sequence of the whole gene synthesis fragment Pcmv-MCP-SV40 pA-Pcmv-ankyrin repeats-SV40 pA, MCP and ankyrin repeats is attached. This fragment is homologous recombination (GBI) with the 2k fragment on the plasmid pBR322. The reaction system is: 15ul GBclonart reaction solution; 1ul pBR322 fragment; 2ul whole gene synthesis fragment; 2ul ddH2O. After incubating at 50°C for 30 min, 5ul was taken to transform Escherichia coli DH5a. Ampicillin LB solid plate screening. The correct clone was verified by sequencing, expanded culture in 500ml LB medium, and a large number of plasmids were extracted for future use. The formula of the liquid LB medium is: 0.5% yeast extract; 1% peptone; 1% sodium chloride, the pH value of the bar is 7.0, sterilized at 121°C for 15 minutes. The solid LB medium is to add 1.5% agar powder on the basis of the liquid LB medium;
[0021] DNA vaccine f...
Embodiment 2
[0023] Embodiment 2: Taking turbot as object's immune protection experiment by injection
[0024] The experimental turbot were randomly divided into 14 groups, each group had 3 tanks, and 10 fish / tank. The prepared DNA vaccine was immunized by intramuscular injection, the injection volume was 1ug / tail and 10ug / tail, and the control was physiological saline. After 4 weeks of immunization, challenge with iridovirus (105CFU / tail). After 15 days, the number of deaths was observed, and the immune protection rate of each group was calculated (see Table 1).
[0025] Table 1
[0026]
[0027] It can be seen from the above that the DNA vaccine has a good control effect on iridescent virus, and the immune protection rate is 82%.
[0028] 1. MCP nucleotide sequence:
[0029]atgtctgcaatctcaggtgcgaacgtaaccagtgggttcatcgacatctccgctttcgatgcgatggagacccacttgtacggcggcgacaatgccgtgacatactttgcccgcgagaccgtgcgtagttcctggtacagcaaactacccgtcaccctgtcaaaacagactggccatgccaa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com