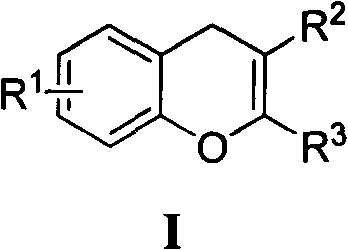
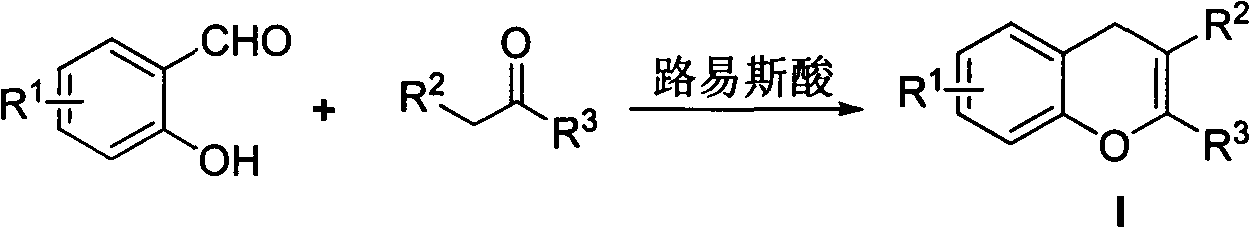
New simple method for synthesizing 4H-benzopyran ring heterocyclic compound
A technology for heterocyclic compounds and benzopyran rings, applied in the field of simple synthesis of 4H-benzopyran ring heterocyclic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
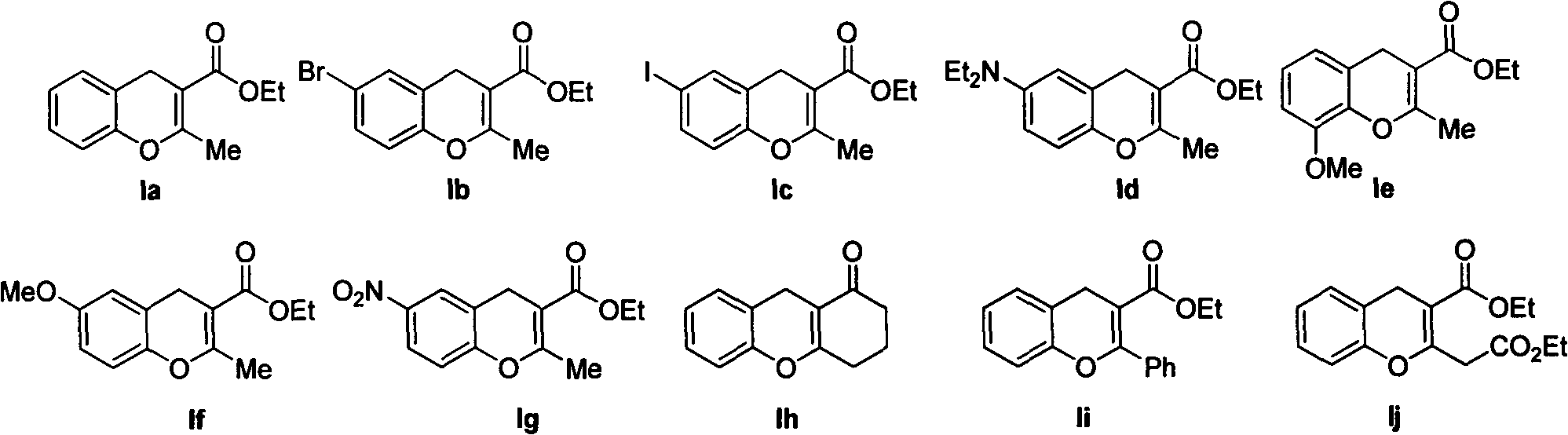
[0018] In the presence of 5 mmol of trimethylsilyl chloride, 1 mmol of salicylaldehyde and 1 mmol of ethyl acetoacetate were reacted at room temperature in 2 milliliters of N, N-dimethylformamide, reacted for 12 hours, and reduced Press spin dry solvent, then column chromatography obtains 2-methyl-4H-benzopyran-3-formic acid ethyl ester Ia (in the formula I, R 1 = H, R 2 = Me,R 3 =CO 2 Et), the yield is 85%.
[0019] 1 H NMR (400MHz, CDCl 3 , TMS) δ1.33(t, J=7.2Hz, 3H), 2.38(s, 3H), 3.60(s, 2H), 4.23(dd, J=7.6, 14.4Hz, 2H), 6.90(dd, J =1.2, 8.4Hz, 1H), 7.02(dt, J=1.2, 7.2Hz, 1H), 7.09-7.16(m, 2H); 13 C NMR (100MHz, CDCl 3 , TMS) δ14.4, 19.2, 24.8, 60.1, 100.9, 116.0, 120.5, 124.0, 127.5, 128.7, 150.1, 160.6, 167.6.
Embodiment 2
[0020] Embodiment 2, its specific synthetic steps refer to embodiment 1.
[0021] 6-bromo-2-methyl-4H-benzopyran-3-carboxylic acid ethyl ester Ib (in formula I, R 1 =6-Br, R 2 = Me,R 3 =CO 2 Et), the yield is 76%.
[0022] 1 H NMR (400MHz, CDCl 3 , TMS) δ1.32(t, J=7.2Hz, 3H), 2.37(s, 3H), 3.57(s, 2H), 4.22(dd, J=6.8, 14.0Hz, 2H), 6.78(d, J =9.2Hz, 1H), 7.23-7.24(m, 2H); 13 C NMR (100MHz, CDCl 3 , TMS) δ14.3, 19.1, 24.6, 60.3, 100.7, 116.2, 117.7, 122.7, 130.4, 131.4, 149.3, 160.3, 167.3.
Embodiment 3
[0023] Embodiment 3, its specific synthetic steps refer to embodiment 1.
[0024] 6-iodo-2-methyl-4H-benzopyran-3-carboxylic acid ethyl ester Ic (in formula I, R 1 =6-I, R 2 = Me,R 3 =CO 2 Et), the yield is 76%.
[0025] yellow liquid; 1 H NMR (400MHz, CDCl 3 , TMS) δ1.32(t, J=7.2Hz, 3H), 2.36(s, 3H), 3.56(s, 2H), 4.23(dd, J=7.2, 14.4Hz, 2H), 6.66(d, J =9.2Hz, 1H), 7.42(s, 2H); 13 C NMR (100MHz, CDCl 3 , TMS) δ14.3, 19.2, 24.4, 60.3, 68.7, 100.9, 118.2, 123.3, 136.4, 137.4, 150.1, 160.3, 167.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com