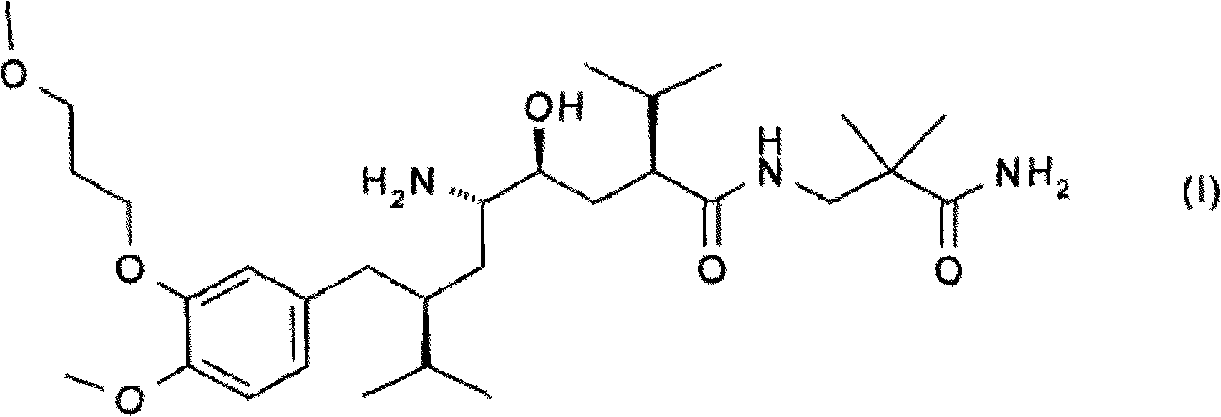
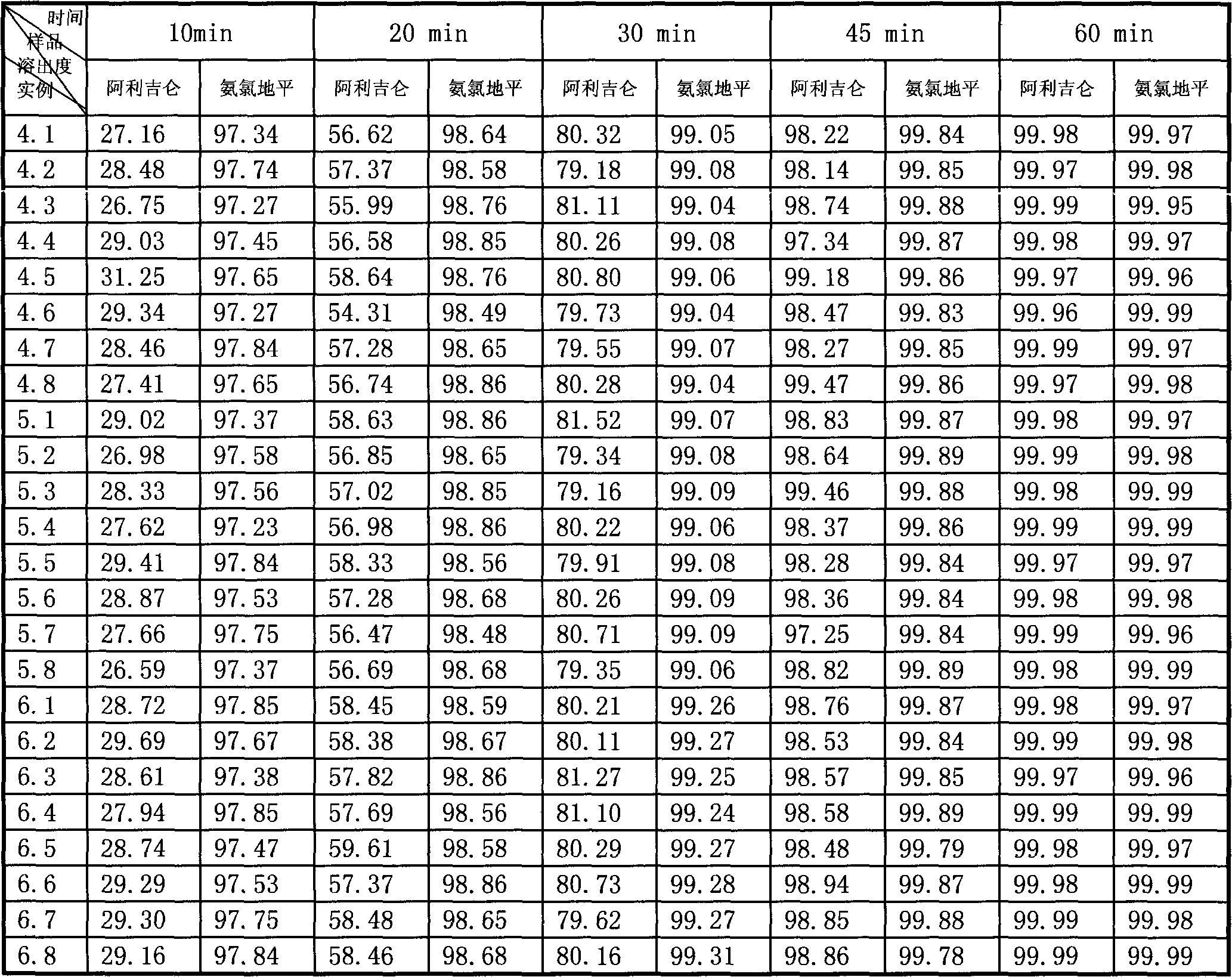
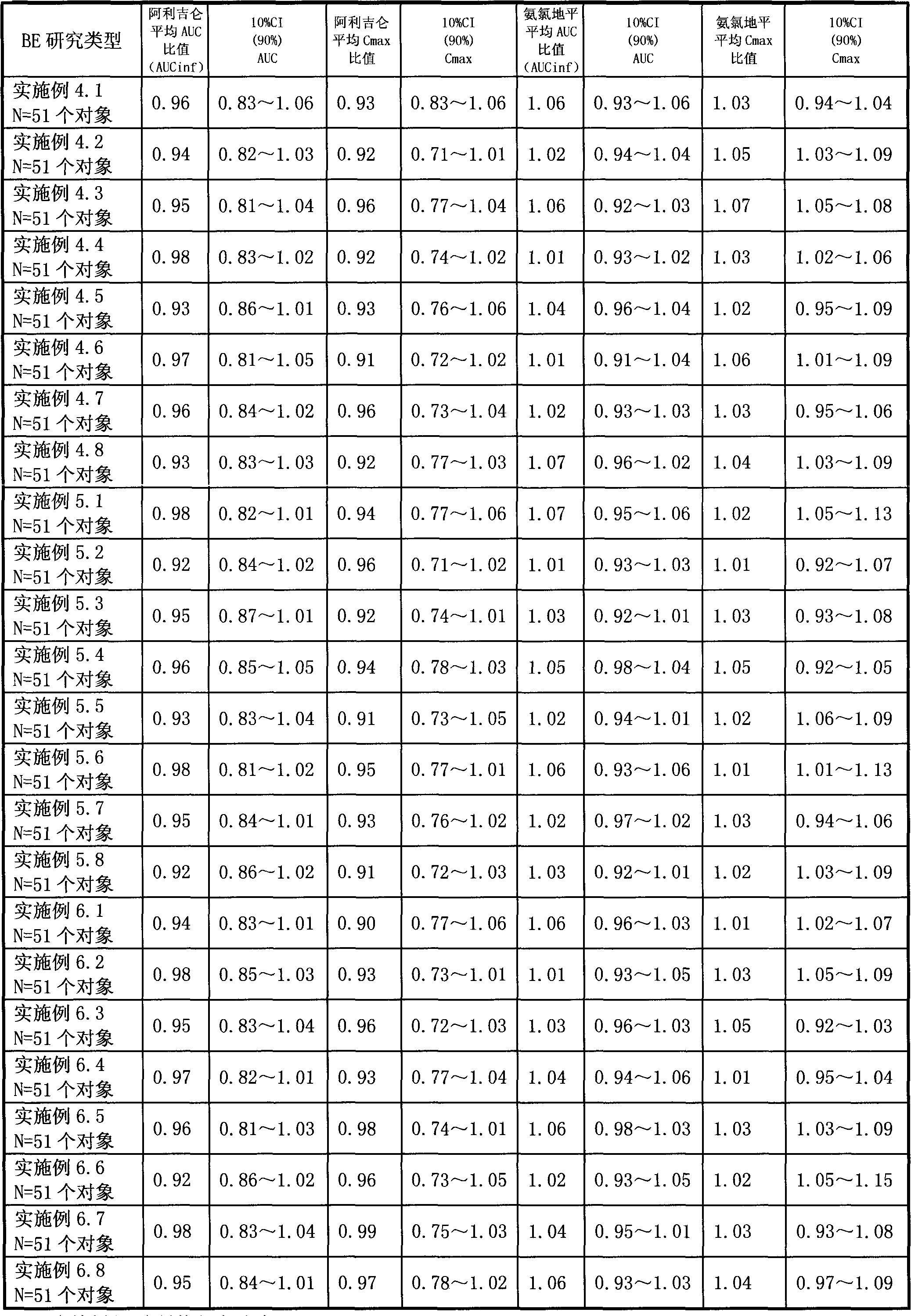
Novel preparation process for oral solid preparation of aliskiren and amlodipine/levamlodipine
A technology of levamlodipine and amlodipine, applied in the field of new methods and medicines, galenic preparations, can solve the problems of reducing patient compliance, difficulty in taking medicine, and rising costs, so as to increase medication compliance, The effect of reducing dosage and reducing tablet weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 Aliskiren Granules
[0058] Take Aliskiren or its pharmaceutically acceptable salt and binder (povidone) according to the ratio (weight ratio) of 8 to 12:1 and mix them uniformly by equal-volume incremental method, and adjust the gap between the two pressure rollers of the dry granulator to be 1mm , the roller pressure is 80~100×100Pa, the screw speed is 80r·min -1 , compaction speed 19r·min -1 , Vacuum ~ 0.4×1.01 5 Pa, choose 16 mesh for the upper screen, choose 18 mesh for the lower screen, and dry granulate. The content of aliskiren (free base) in the granules was determined.
Embodiment 2
[0059] Example Diamlodipine Granules
[0060] Take amlodipine or its pharmaceutically acceptable salt, filler (microcrystalline cellulose), and disintegrant (sodium hydroxyethyl starch) in the ratio (weight ratio) of 1 to 3: 3 to 5: 1 in equal amounts. Addition and mixing evenly, adjust the gap between the two pressure rollers of the dry granulator to 1mm, the pressure of the pressure rollers to 60~80×100Pa, and the screw speed to 80r min -1 , compaction speed 19r·min -1 , Vacuum ~ 0.4×1.01 5 Pa, choose 16 mesh for the upper screen, choose 18 mesh for the lower screen, and dry granulate. The content of amlodipine (free base) in the granules was determined.
Embodiment 3
[0061] Embodiment 3 Levoamlodipine Granules
[0062] Take levamlodipine or its pharmaceutically acceptable salt, filler (microcrystalline cellulose), disintegrant (sodium hydroxyethyl starch) and adopt equal amounts according to the ratio (weight ratio) of 1~3:3~5:1 Mix evenly by incremental method, adjust the gap between the two pressure rollers of the dry granulator to 1mm, the pressure of the pressure rollers to 60~80×100Pa, and the screw speed to 80r min -1 , compaction speed 19r·min -1 , Vacuum ~ 0.4×1.01 5 Pa, choose 16 mesh for the upper screen, choose 18 mesh for the lower screen, and dry granulate. The content of amlodipine (free base) in the granules was determined.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com