Preparation method of ester peptide
A technique for lipopeptides and crude peptides, applied in the field of chemical synthesis of peptides, can solve the problems of difficult control of fermentation process, increased difficulty of purification, and unfavorable industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
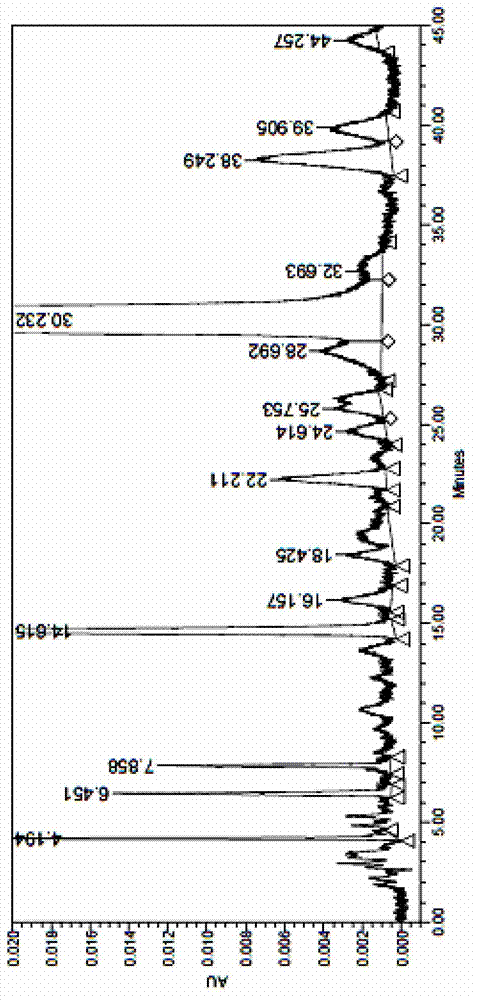
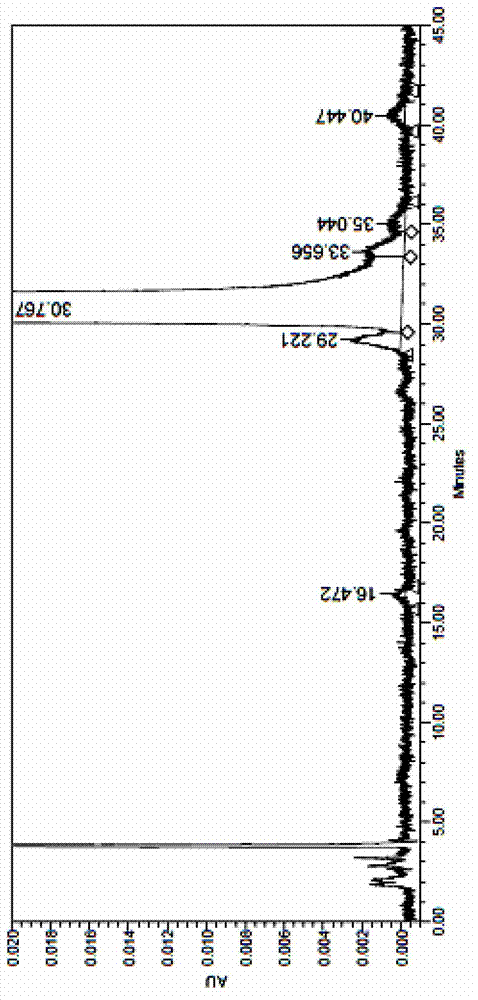
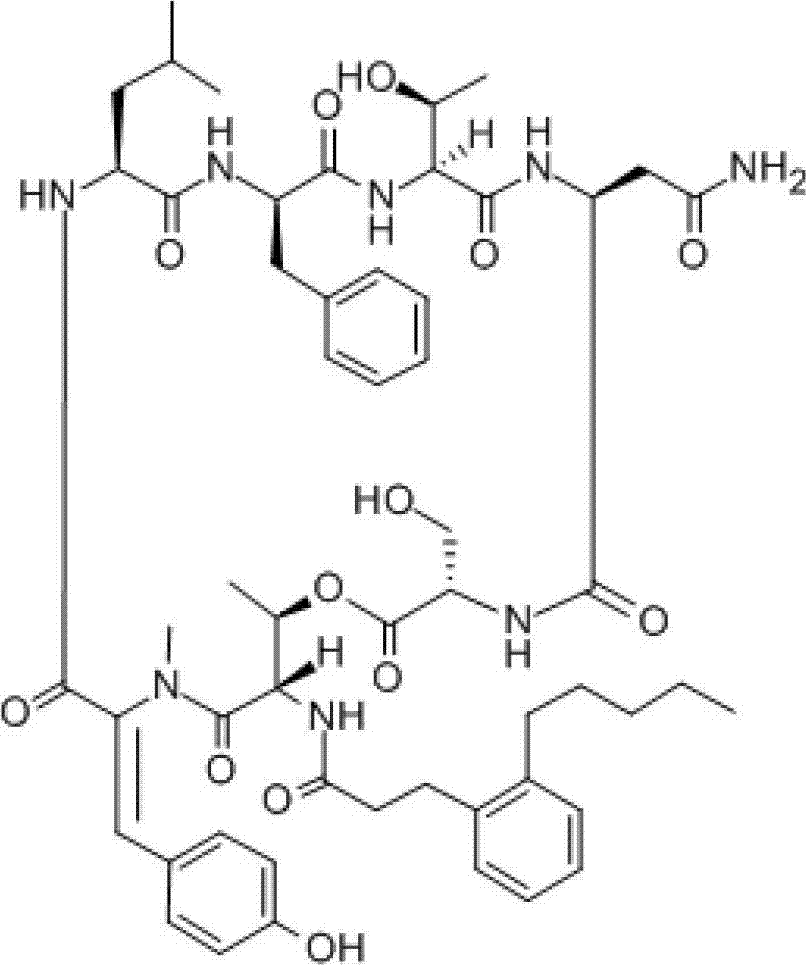
Image
Examples
Embodiment 1
[0110] The connection of embodiment 1Fmoc-Asp-OAll and Rink amide resin
[0111] Put 100g of Rink amide resin (10mmol, substitution degree 0.1mmol / g) into the solid-phase reaction column, wash it twice with DMF, add 1L DMF to swell for 30min. Add 1L of 20% DBLK for deprotection for 10 minutes, add 1L of 20% DBLK for deprotection for 15 minutes, and wash with DMF for 6 times. Dissolve 11.86g of Fmoc-Asp-OAll and 4.26g of HOBt in 100ml of DMF, ice-bath for 10 minutes, add 4.9ml of DIC, pre-activate for 2-5min, add the activated solution to the solid-phase reaction column, stir for 2h, the ninhydrin detection shows feminine. Dried, washed 3 times with DMF, deprotected DBLK twice (5+5min), washed 6 times with DMF, and obtained The resin is ready for use.
Embodiment 2
[0112] The connection of embodiment 2Fmoc-Asp-OAll and Rink amide resin
[0113] Put 10 g (10 mmol, substitution degree 1.0 mmol / g) of Rink amide resin into the solid phase reaction column, wash it twice with DMF, add 100 ml DMF to swell for 30 min. Add 100ml 20% DBLK for deprotection for 10 minutes, add 100ml 20% DBLK for deprotection for 15 minutes, and wash with DMF for 6 times. Dissolve 11.86g of Fmoc-Asp-OAll and 4.26g of HOBt in 60ml of DMF, ice bath for 10 minutes, add 4.9ml of DIC, pre-activate for 2~5min, put the activated solution into the solid-phase reaction column, stir for 2h, the ninhydrin detection shows feminine. Dried, washed 3 times with DMF, deprotected DBLK twice (5+5min), washed 6 times with DMF, and obtained The resin is ready for use.
Embodiment 3
[0114] The connection of embodiment 3Fmoc-Asp-OAll and Rink amide resin
[0115] Put 20g of Rink amide resin (10mmol, substitution degree 0.5mmol / g) into the solid-phase reaction column, wash it twice with DMF, add 200ml DMF to swell for 30min. Add 200ml 20% DBLK for deprotection for 10 minutes, add 200ml 20% DBLK for deprotection for 15 minutes, and wash with DMF for 6 times. Dissolve 11.86g of Fmoc-Asp-OAll and 4.26g of HOBt in 60ml of DMF, ice bath for 10 minutes, add 4.9ml of DIC, pre-activate for 2~5min, put the activated solution into the solid-phase reaction column, stir for 2h, the ninhydrin detection shows feminine. Dried, washed 3 times with DMF, deprotected DBLK twice (5+5min), washed 6 times with DMF, and obtained The resin is ready for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com