Vesicle composition, and external skin preparation and cosmetic, each containing same
A technology of composition and vesicles, applied in the field of skin external preparations and cosmetics, can solve problems such as long-term stability limitation, and achieve the effect of excellent long-term stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
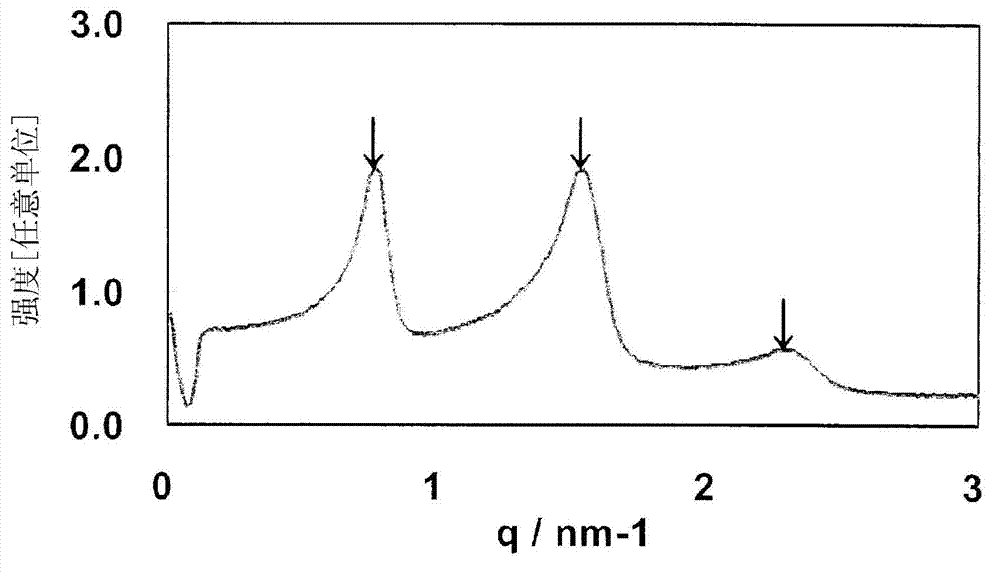
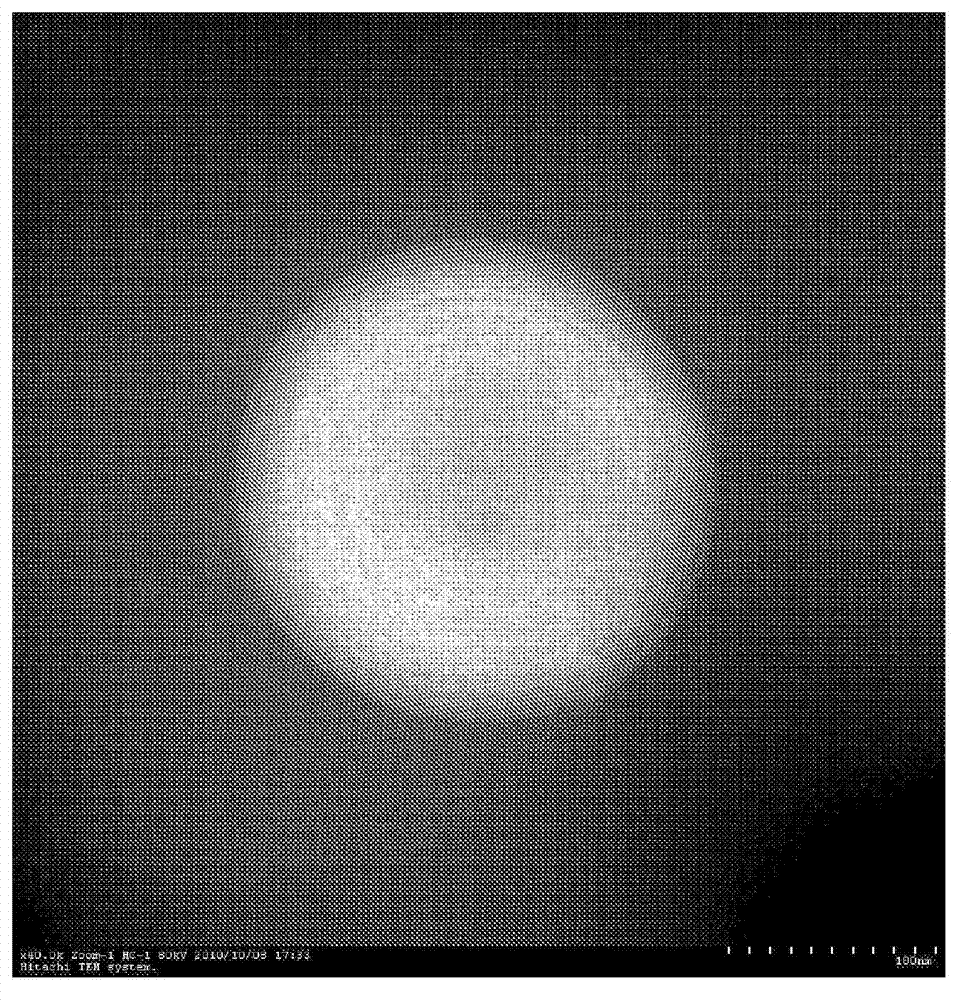
Image
Examples
preparation example Construction
[0078]The method for preparing the vesicle composition of the present invention is not particularly limited, and it can be prepared by a common method. For example, it can be prepared by the following methods: vortexing method [A.D.Bangham, J.Mol.Biol., 13, 238 (1965)], sonication method [C.Huang, Biochem., 8, 344 (1969)], pretreatment Vesicle method (prevesicle method) [H. Trauble, Neurosci. Res. Prog. Bull., 9, 273 (1971)], ethanol injection method [S. Batzri, Biochem. Biophys. French Crusher extrusion method [Y.Barenholz, FEBS Lett., 99,210(1979)], bile acid removal method [Y.Kagawa, J.Biol.Chem., 246,5477(1971)], TritonX-100 Batch method [W.J.Gerritsen, Eur.J.Biochem., 85,255 (1978)], Ca 2+ Fusion method [D.Papahadojopoulos, Biochem.Biophys.Acta., 394,483 (1975)], ether injection method [D.Deazer, Biochem.Biophys.Acta., 443,629 (1976)], annealing method [R.Lawaczeck, Biochem. Biophys.Acta., 443,313(1976)], freeze-thaw fusion method [M.Kasahara, J.Biol.Chem., 252,7384(197...
Embodiment
[0104] Hereinafter, examples and test examples are given to specifically describe the present invention, but the present invention is not limited to these examples and the like.
[0105] [Vesicle composition of Examples 1 to 15]
[0106] Using the components shown in the columns of Examples 1 to 15 in Table 1, a vesicle composition was prepared by the following production method.
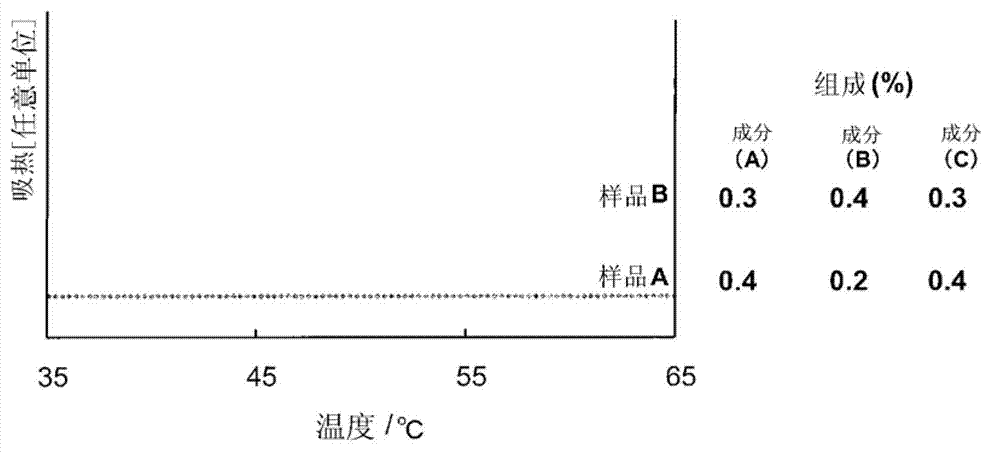
[0107] In addition, for the vesicle composition of Example 13 ( image 3 Sample B), except changing the vesicle composition of Example 2 ( image 3 Sample A) was prepared in the same manner as in Example 2 except for the contents of component (A) polyoxyethylene (5 mol) phytosterol, component (B) batyl alcohol, and component (C) cholesterol.
[0108] Step 1: The components selected from components 1 to 17 are heated to 95° C. to form a solution.
[0109] Step 2: Purified water was added to the solution obtained in Step 1 while maintaining the temperature at 75° C., and a dispersion liquid was obt...
Embodiment 16
[0160] Embodiment 16: ointment
[0161] The ointment is prepared by the following ingredients and production method.
[0162]
[0163] (Note 1) Manufactured by Wako Pure Chemical Industries, Ltd.
[0164] (Note 2) Manufactured by Eisai Co., Ltd.
[0165] (Production method)
[0166] A: A part of ingredients (3), (4) and (9) was heated and mixed, and kept at 75°C.
[0167] B: Components (1), (2), (7), and (8) were heated and mixed, and kept at 75°C.
[0168] C: B is slowly added to A, and (5) dissolved with the remainder of component (9) is added while cooling, and component (6) is further added to obtain an ointment.
[0169] The ointment of Example 16 was excellent in the stability of the vesicle composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com