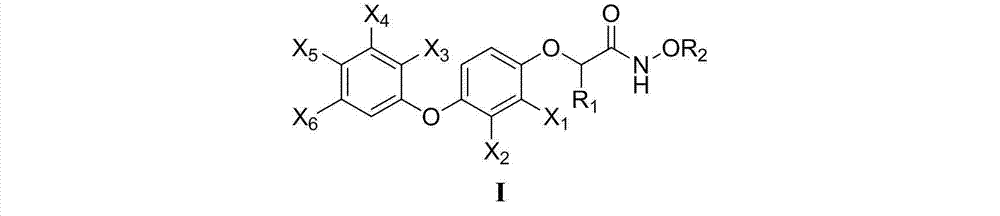
2-(4-aryloxyphenoxy)alkylamide and application thereof
A technology of aryloxyphenoxy and alkamide, applied in the direction of nitrile/isonitrile active ingredients, organic chemistry, drug combination, etc., can solve the problem of 2-(4-aryloxyphenoxy)alkanamide or its optical There are no research and development reports on the anticancer activity of isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
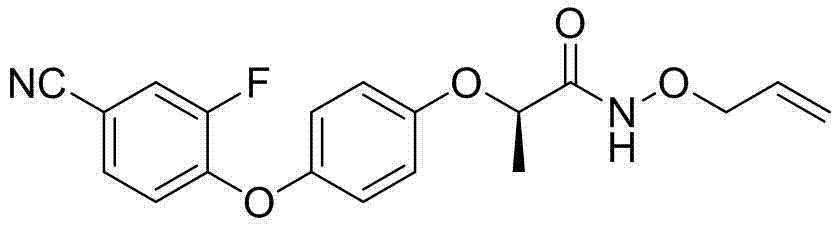
[0018] Preparation of (R)-N-propargyloxy-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionamide
[0019]
[0020] (1) Preparation of (R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionic acid
[0021] N,N-Dimethylformamide (DMF, 40 mL), (R)-2-(4-hydroxyphenoxy)propionic acid (3.64 g, 0.02 mol), and potassium carbonate (5.41 g 0.04 mol) were added in portions , stirred at 70-80 °C for 1 h, added a DMF solution of 3,4-difluorobenzonitrile (2.78 g, 0.02 mol) dropwise, and continued stirring for 5-6 h. Cool to room temperature, pour into ice water (250 mL), slowly add dilute hydrochloric acid, adjust to pH 4~5, filter, wash with water, and dry to obtain 5.20 g of the title compound as a gray solid, with a yield of 86.4%.
[0022] (2) Preparation of (R)-N-propargyloxy-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionamide
[0023] Toluene (40 mL), (R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propanoic acid (1.01 g, 3.3 mmol) and thionyl chloride (1.18 g, 10 mmol). Reflux for 5 h, remove ...
Embodiment 2
[0025] Preparation of (R)-N-(3,3-dichloro-2-allyloxy)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionamide
[0026]
[0027] (R)-N-(3,3-dichloro-2-allyloxy)-2-[4-(4-cyano-2-fluorophenoxy) of white solid was obtained according to the method of Example 1 Phenoxy]propionamide. Melting point 92.2~92.7 ℃, 1 H NMR (300 MHz, CDCl 3 ) δ: 1.62 (d, J=6.6 Hz, 3H, CHC H 3 ), 4.55 (d, J=6.9 Hz, 2H, OCH 2 ), 4.73 (q, J=6.6 Hz, 1H, C H CH 3 ), 6.14 (t, J=7.2 Hz, 1H, =CH), 6.87~7.49 (m, 7H, C 6 h 4 , C 6 h 3 ), 8.93 (s, 1H, NH); LC-MS (Pos M + ) m / z): 425.
Embodiment 3
[0029] Preparation of (R,Z)-N-(3-chloroallyloxy)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionamide
[0030]
[0031](R, Z)-N-(3-chloro-allyloxy)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy was obtained as a white solid by the method of Example 1 ] Propionamide. Melting point 95.2~96.3 ℃, 1 H NMR (300 MHz, CDCl 3 ) δ: 1.61 (d, J=6.6 Hz, 3H, CHC H 3 ), 4.67 (d, J=6.6 Hz, 2H, OCH 2 ), 4.74 (q, J=6.9 Hz, 1H, C H CH 3 ), 6.02~6.08 (m, 1H, OCH 2 C H ), 6.30 (d, J=7.5 Hz, 1H, =CHCl), 6.87~7.49 (m, 7H, C 6 h 4 , C 6 h 3 ), 8.93 (s, 1H, NH); LC-MS (Pos M + ) m / z): 391.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com