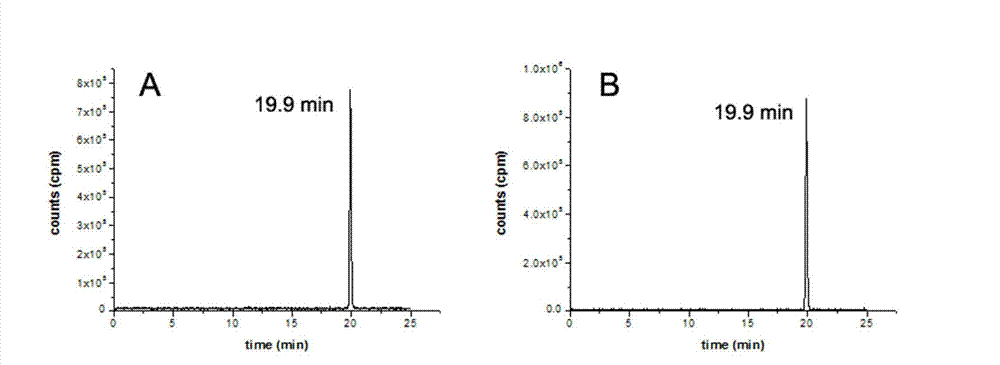
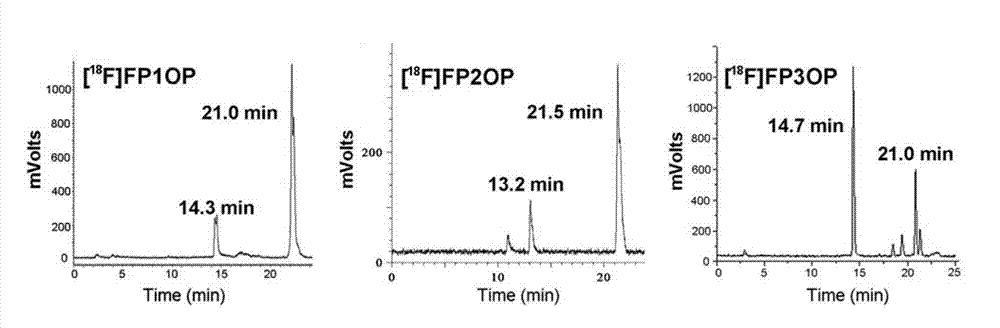
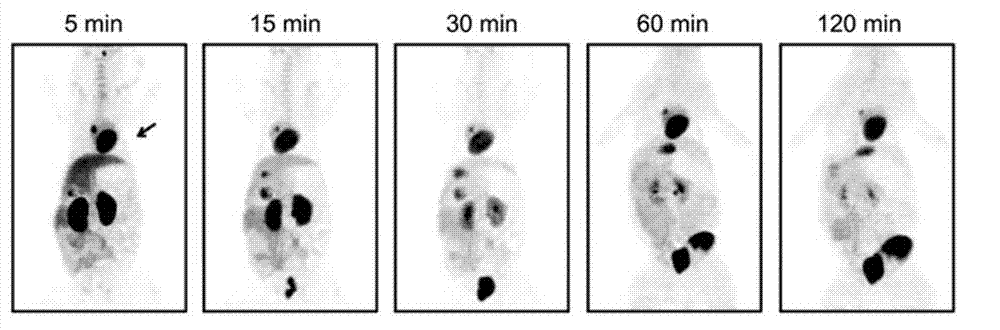
PEGylated benzyltriazolyl pyridazinone compounds, and preparation method and application thereof
A technology for compounding benzyltriazolyl and pyridazinone, which is applied in the field of pyridazinone compounds containing PEGylated benzyltriazolyl and its preparation, can solve the problems of slow metabolism, fast clearance, poor stability, etc., and achieve Wide range of sources, favorable yield, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Synthesis of ligand mpp-nOTs (n=1):
[0056] a. Synthesis of furochloric acid (DCA)
[0057] Under ice bath, add 480mL of concentrated hydrochloric acid into a 1L four-neck flask. At 0-10°C, add 80g MnO in batches 2 , stirred, and slowly added dropwise 24g furfural. After stirring at room temperature for 30 min, the temperature was slowly raised to 60°C. At 60-80°C, add 42g MnO in batches 2 , heated up to 100°C. After the reaction until the liquid turned into an orange transparent solution, the reaction was continued for 30 min, cooled, and suction filtered. The precipitate was dissolved with 40 mL of ether and filtered. The filtrate was evaporated to remove the solvent, and recrystallized with 40 mL of water to obtain 31 g of furochloric acid as light pink flaky crystals. NMR spectrum: ( 1 HNMR, CDCl 3 )δ:6.085(s,1H,HO-C-H);( 13 CNMR, CDCl 3 ) δ: 98.56, 123.87, 151.25, 165.15. Infrared Spectrum: (IR) / cm -1 : vOH:3407, vC=O:1770, vC=C:1638.
[0058] b....
Embodiment 2
[0078] (1) Synthesis of ligand mpp-nOTs (n=2):
[0079] a. Synthesis of furochloric acid (DCA): same as Example 1.
[0080] b. Synthesis of 2-tert-butyl-4,5-dichloropyridazinone (DCP): Same as Example 1.
[0081] c. Synthesis of mIP-OH: Same as Example 1.
[0082] d. Synthesis of mIP-OTs: Same as Example 1.
[0083] e.mIP-N 3 Synthesis: with embodiment 1.
[0084] f. Synthesis of P-nOH (n=2)
[0085] Under nitrogen protection at 0°C, 20 mmol of diethylene glycol was dissolved in redistilled THF, and 0.31 g (13 mmol) of NaH was slowly added. After stirring for 30 minutes, slowly inject 1.98g of 80% 3-bromopropyne in toluene and stir for 2 hours. Stir at room temperature for 20 h, pour the reaction solution into water, extract with 100 mL of dichloromethane, and wash the organic phase with anhydrous Na 2 SO 4 Dry and spin dry to obtain P-nOH (n=2). NMR spectrum: ( 1 HNMR, CDCl 3 )δ:2.164(s,1H,O-H),2.452(t,1H,C≡CH),3.612-3.764(m,8H,(OCH 2 CH 2 ) 2 ),4.221(d,2H,C≡CCH...
Embodiment 3
[0096] (1) Synthesis of ligand mpp-nOTs (n=3):
[0097] a. Synthesis of furochloric acid (DCA): same as Example 1.
[0098] b. Synthesis of 2-tert-butyl-4,5-dichloropyridazinone (DCP): Same as Example 1.
[0099] c. Synthesis of mIP-OH: Same as Example 1.
[0100] d. Synthesis of mIP-OTs: Same as Example 1.
[0101] e.mIP-N 3 Synthesis: with embodiment 1.
[0102] f. Synthesis of P-nOH (n=3)
[0103] Under nitrogen protection at 0°C, 20 mmol of triethylene glycol was dissolved in redistilled THF, and 0.31 g (13 mmol) of NaH was slowly added. After stirring for 30 minutes, slowly inject 1.98g of 80% 3-bromopropyne in toluene and stir for 2 hours. Stir at room temperature for 20 h, pour the reaction solution into water, extract with 100 mL of dichloromethane, and wash the organic phase with anhydrous Na 2 SO 4 Dried and spin-dried to obtain P-nOH (n=3). NMR spectrum: ( 1 HNMR, CDCl 3 )δ:2.357(s,1H,O-H),2.442(t,1H,C≡CH),3.609-3.749(m,12H,(OCH 2 CH 2 ) 3 ),4.215(d,2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com