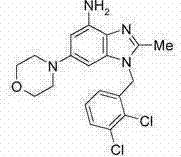
Benzimidazole derivatives as pi3 kinase inhibitors
A technology of benzimidazole and compounds, applied in the field of benzimidazole derivatives, can solve problems such as unidentified somatic mutations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0344]
[0345] Preparation of 5-bromo-2-methyl-7-nitro-1H-benzimidazole
[0346] a) 4-bromo-2,6-dinitroaniline
[0347]
[0348] Bromine (1.5 mL, 30 mmol) was added dropwise to a stirred suspension of 2,6-dinitroaniline (5 g, 27.3 mmol) in glacial acetic acid (50 mL) and heated at 120 °C for 2 h. After cooling to ambient temperature, the resulting mixture was poured into water (50 mL). The precipitated solid was collected by filtration, washed with water, and dried in vacuo. The solid was redissolved in EtOAC, washed with water and saturated brine. The organic layer was collected and concentrated in vacuo to give the desired product (6.88 g, 95%). 1 HNMR (300MHz, DMSO-d 6 ) δppm 8.37 (brs, 2H), 8.58 (s, 2H).
[0349] b) 5-bromo-3-nitrobenzene-1,2-diamine
[0350]
[0351] 4-Bromo-2,6-dinitroaniline was dissolved in EtOH (50 mL), and (NH 4 ) 2 S (2.2 mL) was added to the mixture. The reaction mixture was heated to 90 °C for 1 h. Thin layer chromatography (...
Embodiment 2
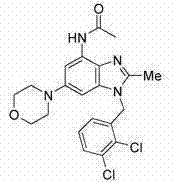
[0355] Example 2 (R=H) and Example 3 (R=Ac)
[0356]
[0357] 2-Methyl-6-(4-morpholinyl)-1-(1-naphthylmethyl)-1H-benzimidazol-4-amine and N-(2-methyl-6-morpholinyl-4 Preparation of -yl-1-naphthalen-1-ylmethyl-1H-benzimidazol-4-yl)-acetamide
[0358] a) 6-bromo-2-methyl-1-(naphthalene-1-ylmethyl)-4-nitro-1H-benzo[d]imidazole
[0359]
[0360] 6-bromo-2-methyl-4-nitro-1H-benzo[d]imidazole (prepared according to the same operation as in Example 1) (3g), 1-(bromomethyl)naphthalene (2.85g ) and K 2 CO 3 (3.23 g) in DMF (100 mL) was stirred overnight at 80°C. It was cooled to room temperature and filtered. Then pour the filtrate into water. It was then filtered to give a solid which was washed with water and dried in vacuo to give the desired product (4.63 g, 100%). 1 HNMR (300MHz, DMSO-d 6 )δppm2.54(s,3H),6.16(s,2H),6.32(d,1H, J =7.5Hz),7.33(t,1H, J =7.5Hz),7.61-7.72(m,2H),7.87(d,1H, J =7.5Hz),8.01(d,1H, J =7.5Hz),8.14(d,1H, J =1.8Hz),8.19(d,1H, J =7.5Hz),8.28...
Embodiment 4
[0367]
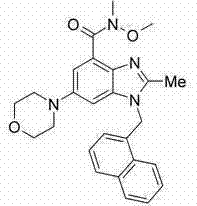
[0368] Preparation of N-[2-methyl-6-(4-morpholinyl)-1-(1-naphthylmethyl)-1H-benzimidazol-4-yl]methanesulfonamide
[0369] a) 2-methyl-6-morpholino-1-(naphthalene-1-ylmethyl)-1H-benzo[d]imidazol-4-amine
[0370]
[0371] 4-(2-Methyl-3-(naphthalen-1-ylmethyl)-7-nitro-3H-benzo[d]imidazol-5-yl)mol, prepared as described in Example 2 morphine (804mg), iron powder (168mg) and FeSO 4 (84mg) in ethanol (30mL) and H 2 The mixture in O (30 mL) was stirred overnight at reflux temperature. The mixture was cooled to room temperature, and the solvent was removed in vacuo. The residue was dissolved in DCM and filtered. Then the filtrate was washed with brine, washed with anhydrous Na 2 SO 4 Drying, filtering, and concentration in vacuo afforded the desired product as a solid (720 mg, 97%). 1 HNMR (300MHz, DMSO-d 6 )δppm2.33(s,3H),2.91(t,4HJ=4.8Hz),3.64(t,4HJ=4.8Hz),5.15(brs,2H),5.83(s,2H),6.10(d,1H, J=2.1Hz),6.12(d,1H,J=2.1Hz),6.38(d,1H,J=7.5Hz),7.34(t,1H,J=7.5Hz),7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com