Method for increasing hydrogen desorption capacity by sodium borohydride through hydrolysis
A technology of sodium borohydride water and sodium borohydride, which is applied in the field of increasing the amount of hydrogen liberated by sodium borohydride hydrolysis, can solve the problems of reduced catalyst area, easy agglomeration of hydrolyzed products, and limited Co-B catalyst performance, so as to improve the release rate. The effect of the amount of hydrogen and the method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] A method for improving the amount of hydrogen released by hydrolysis of sodium borohydride, the steps are as follows: 1) 1.07gNaBH 4 with 0.101g CoCl 2 Add 4g of water, carry out hydrolysis reaction at 25-30°C for 2-5 minutes, then filter to obtain the filter residue, dry the filter residue to obtain the Co-B catalyst, mix 0.004g of Co-B catalyst with 0.01g of CeO 2 Stir evenly with a glass rod in a mortar to obtain a mixed catalyst; 2) Make NaOH and water into a strong alkali solution with a pH value of 12-13, and then hydroborate 0.0178g weighed in a glove box filled with argon Sodium is added to the strong alkali solution and mixed evenly to obtain a sodium borohydride alkaline aqueous solution. The mass fraction of sodium borohydride in the sodium borohydride alkaline aqueous solution is 9%, and finally the mixed catalyst is added to the sodium borohydride alkaline aqueous solution for hydrolysis. hydrogen reaction.
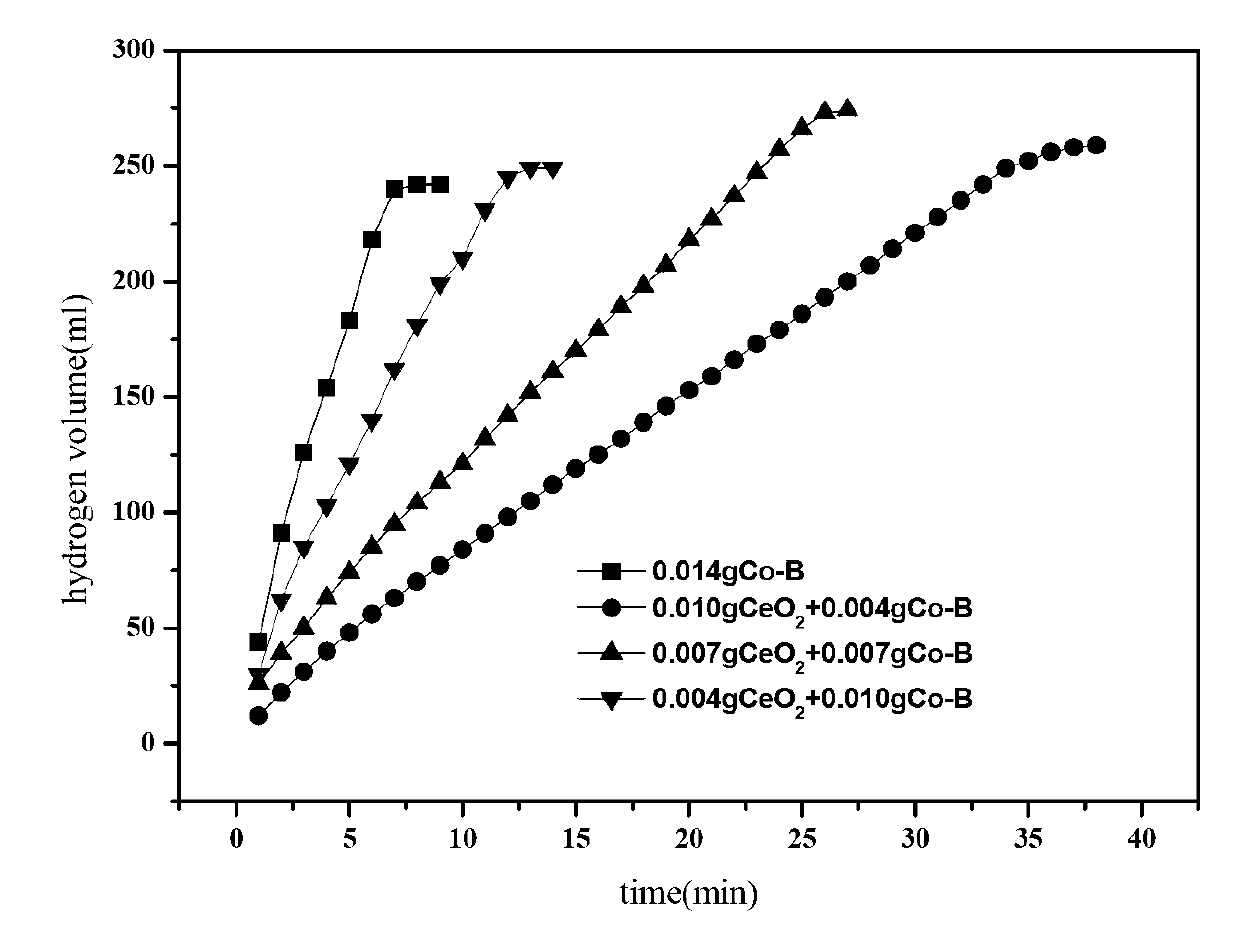
[0015] From figure 1 It can be seen that the ...
Embodiment 2
[0018] A method for improving the amount of hydrogen released by hydrolysis of sodium borohydride, the steps are as follows: 1) 1.07gNaBH 4 with 0.101g CoCl 2 Add 4g of water, carry out hydrolysis reaction at 25-30°C for 2-5 minutes, then filter to obtain the filter residue, dry the filter residue to obtain the Co-B catalyst, mix 0.007g of Co-B catalyst with 0.007g of CeO 2 Stir evenly with a glass rod in a mortar to obtain a mixed catalyst; 2) Make NaOH and water into a strong alkali solution with a pH value of 12-13, and then hydroborate 0.0178g weighed in a glove box filled with argon Sodium is added to the strong alkali solution and mixed evenly to obtain a sodium borohydride alkaline aqueous solution. The mass fraction of sodium borohydride in the sodium borohydride alkaline aqueous solution is 9%, and finally the mixed catalyst is added to the sodium borohydride alkaline aqueous solution for hydrolysis. hydrogen reaction.
[0019] From figure 1 It can be seen that the...
Embodiment 3
[0022] A method for improving the amount of hydrogen released by hydrolysis of sodium borohydride, the steps are as follows: 1) 1.07gNaBH 4 with 0.101g CoCl 2 Add 4g of water, carry out hydrolysis reaction at 25-30°C for 2-5 minutes, then filter to obtain the filter residue, dry the filter residue to obtain the Co-B catalyst, mix 0.01g of Co-B catalyst with 0.004g of CeO 2 Stir evenly with a glass rod in a mortar to obtain a mixed catalyst; 2) Make NaOH and water into a strong alkali solution with a pH value of 12-13, and then hydroborate 0.0178g weighed in a glove box filled with argon Sodium is added to the strong alkali solution and mixed evenly to obtain a sodium borohydride alkaline aqueous solution. The mass fraction of sodium borohydride in the sodium borohydride alkaline aqueous solution is 9%, and finally the mixed catalyst is added to the sodium borohydride alkaline aqueous solution for hydrolysis. hydrogen reaction.
[0023] From figure 1 It can be seen that the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com