Amino acid derivatives of seedvax and application for being used as bactericides thereof
A technology of amino acids and derivatives, which is applied in the direction of fungicides, biocides, and chemicals for biological control, etc., and can solve problems such as environmental problems, increased drug costs, difficult-to-control vascular diseases and root diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
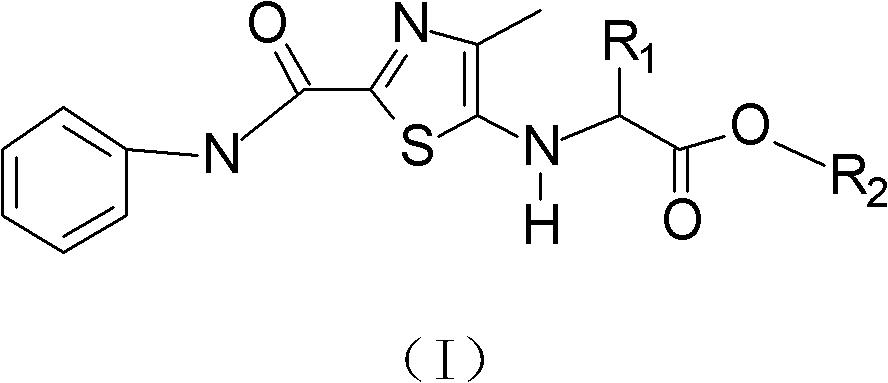
[0022] Synthesis of Glycine Derivatives of Seed Dressing, namely: Synthesis of 2-[(4'-methyl-2'-phenylcarbamoyl)-thiazol-5'-yl]-aminoacetic acid.
[0023] The synthetic compound of present embodiment 1, its structural formula (I-I) is as follows:
[0024]
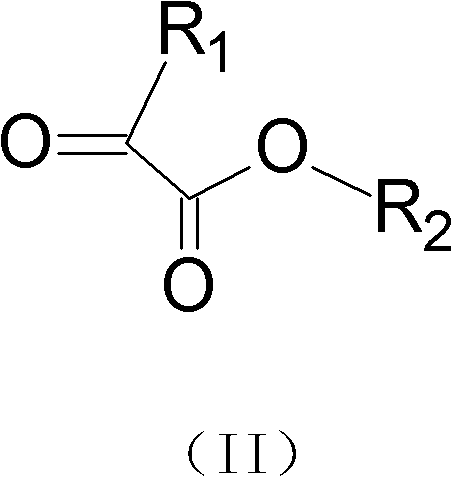
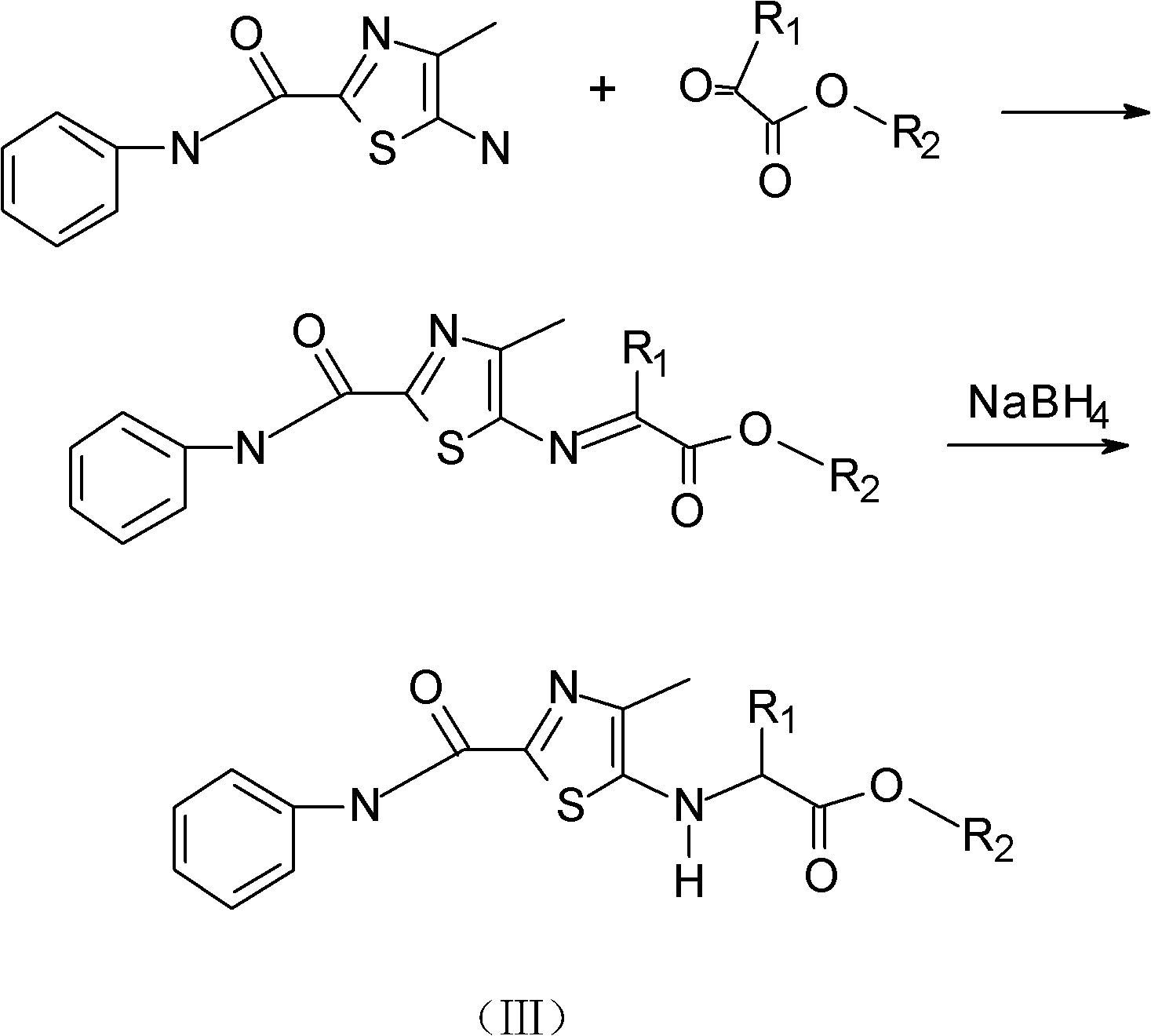
[0025] The specific synthesis steps are as follows: Weigh 7g (0.03mol) of the original drug of Zhuanglingling into a round-bottomed flask, dissolve it in tetrahydrofuran, add 0.03mol of ethyl glyoxylate, stabilize the temperature at 50-70°C, and reflux for 3- After 4 hours, TLC followed the progress of the reaction. After the reaction is complete, add 0.05 mol of sodium borohydride, control the temperature at 25-30°C for 4-5 hours, and track the reaction progress by thin-layer chromatography. After the reaction was complete, the solvent tetrahydrofuran was distilled off. Separation and purification by column chromatography gave 6.4 g of the target product with a yield of 73%.
[0026] target compound 1 HNMR (400MHz, ...
Embodiment 2
[0028] Synthesis of seed dressing alanine derivatives, namely: 2-[(4'-methyl-2'-phenylcarbamoyl)-thiazol-5'-yl]-alanine synthesis.
[0029] The synthetic compound of present embodiment 2, its structural formula (I-II) is as follows:
[0030]
[0031] The specific steps are: Weigh 7 g (0.03 mol) of the original drug of Zhuang Ling into a round-bottomed flask, dissolve it in tetrahydrofuran, add 0.03 mol of ethyl pyruvate, stabilize the temperature at 50-70 ° C, and reflux for 5 hours. Layer chromatography followed the progress of the reaction. After the reaction is complete, 0.05 mol of sodium borohydride is added, the temperature is controlled at 25-30° C. for 5 hours, and the reaction progress is tracked by thin-layer chromatography. After the reaction was complete, the solvent tetrahydrofuran was distilled off. Separation and purification by column chromatography gave 6.4 g of the target product with a yield of 73%.
[0032] target compound 1 HNMR (400MHz, CD 3 Cl) δ...
Embodiment 3
[0034] Biological Activity Test:
[0035] The mycelium growth rate inhibition method was used to measure the indoor antibacterial activity of the seed-dressing spirit amino acid derivatives against rice sheath blight, and the seed-dressing spirit original drug was used as a contrast, and the inhibitory rate of the medicinal liquid to the growth of mycelia was calculated according to the following formula. EC of pesticides against rice sheath blight 50 .
[0036]
[0037] The results are listed in Table 1. It can be seen from the results in the table that after the amino acid functional group is derived from Seed Dressing Ling, it still maintains the same bactericidal activity. With the increase of the substituents on the amino acid functional group, the activity tends to decline.
[0038] Table 1: The results of the determination of the bactericidal activity of the amino acid derivatives of Zongling and Zongling
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com