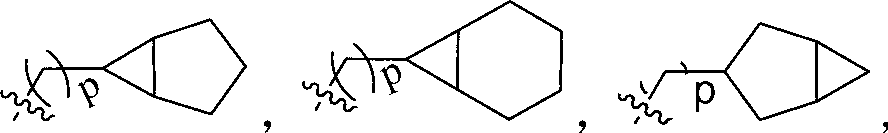
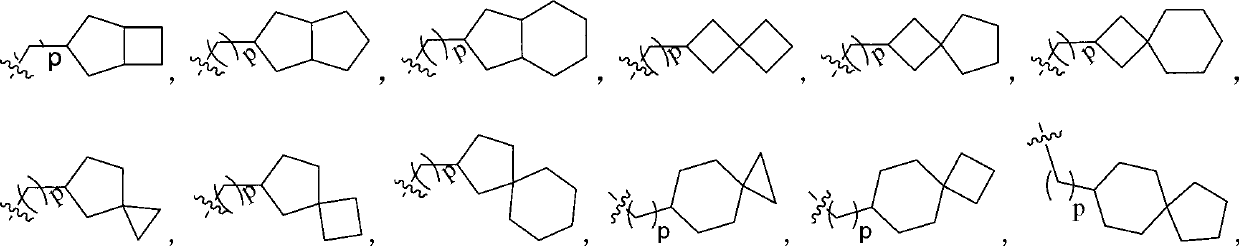
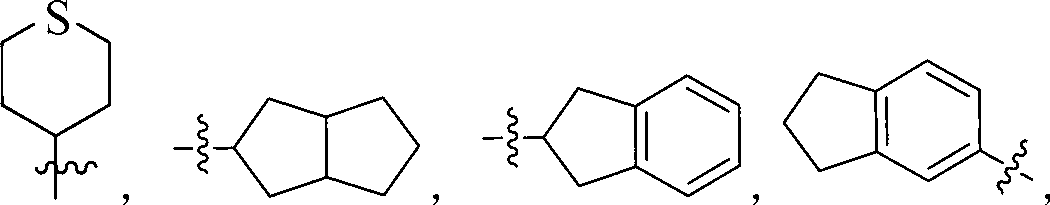
Indolinone derivatives serving as tyrosine kinase inhibitors
A technology of amino compounds, applied in the field of indolinone derivatives as tyrosine kinase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0167] The preparation of step 1 intermediate 1
[0168] Dissolve raw material 1 and organic base in DCM, add raw material 2 dropwise under ice-water bath, raise to room temperature and react for half an hour, add water, extract with DCM, dry, evaporate to dryness, and dry the solid in vacuo to obtain intermediate 1.
[0169] Step 2 Preparation of Intermediate 2
[0170] Dissolve intermediate 1 and organic base in DCM, add raw material 3 dropwise, react at room temperature for 12 h, extract with DCM (dichloromethane), dry the organic layer with anhydrous sodium sulfate, and evaporate to dryness to obtain intermediate 2.
[0171] Step 3 Preparation of Intermediate 3
[0172] Dissolve intermediate 2 in DCM, add TFA, after the reaction at room temperature, concentrate to obtain intermediate 3 or dissolve intermediate 2 in methanol, hydrogenate Pd / C overnight, filter, and concentrate to obtain intermediate 3, the product It was directly used in the next reaction without purifica...
Embodiment 1
[0247] Example 1 (Z)-N-methyl-2-(3-methyl-3-azabicyclo[3.1.0]hexane-6-yl)-N-(4-((2-oxo- 6-(1H-pyrazol-5-yl) Preparation of Indolin-3-ylmethylene)(phenyl)methylamino)phenyl)acetamide
[0248]
[0249] (1) Preparation of 2-chloro-N-methyl-N-(4-nitrophenyl)acetamide
[0250]
[0251] N-Methyl-4-nitroaniline (7.6g, 50mmol) and triethylamine (5.5g, 54mmol) were dissolved in 200mL DCM, and chloroacetyl chloride (11.5g, 50mmol) was added dropwise under ice-water bath, rising to React at room temperature for half an hour, add 30 mL of water, extract DCM, dry, evaporate to dryness, and dry the solid in vacuum to obtain 9.69 g of 2-chloro-N-methyl-N-(4-nitrophenyl)acetamide, with a yield of 85% .
[0252] (2) Preparation of N-methyl-2-(3-methyl-3-azabicyclo[3.1.0]hexane-6-yl)-N-(4-nitrophenyl)acetamide
[0253]
[0254] 2-Chloro-N-methyl-N-(4-nitrophenyl)acetamide (4.56g, 20mmol) and triethylamine (2.42g, 24mmol) were dissolved in 50mL DCM, and 3-methyl -3-Azabicyclo[3....
Embodiment 2
[0286] Example 2 (Z)-N-methyl-2-(3-methyl-3-azabicyclo[3.1.0]hexane-6-yl)-N-(4-((2-oxo- 6-(thiazol-2-yl)indole Preparation of Indoline-3-ylmethylene)(phenyl)methylamino)phenyl)acetamide
[0287]
[0288] (1) (Z)-N-methyl-2-(3-methyl-3-azabicyclo[3.1.0]hexane-6-yl)-N-(4-((2-oxo- 6-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-3-ylmethylene)(phenyl)methylamino)phenyl)-acetamide
[0289]
[0290] (Z)-N-(4-((6-bromo-2-oxoindolin-3-ylmethylene)(phenyl)methylamino)phenyl)-N-methyl-2 -(3-Methyl-3-azabicyclo[3.1.0]hexan-6-yl)acetamide (11.1g, 20mmol) was dissolved in 120mL of dioxane, and bis-pinacol borate was added (7.38g, 29mmol), potassium acetate (2.45g, 25mmol) and Pd(Ph 3 P) 2 Cl 2 (0.7 g, 0.1 mmol). Heat to 90°C overnight. Add water and extract with ethyl acetate, dry, concentrate and separate through silica gel column to obtain (Z)-N-methyl-2-(3-methyl-3-azabicyclo[3.1.0]hexane-6-yl) -N-(4-((2-oxo-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-3-ylmethylene)(benzene B...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell concentration | aaaaa | aaaaa |
| Cell concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com