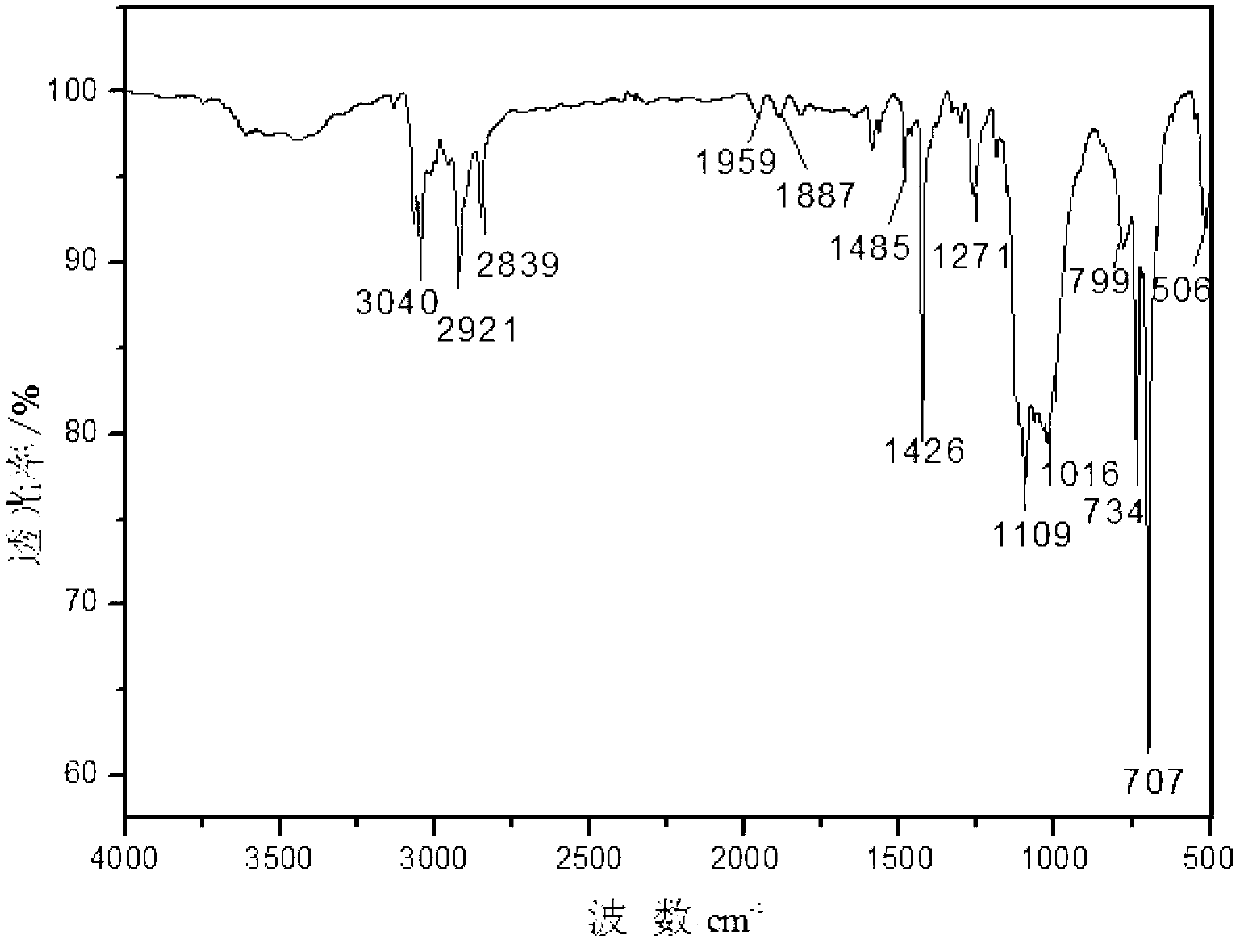
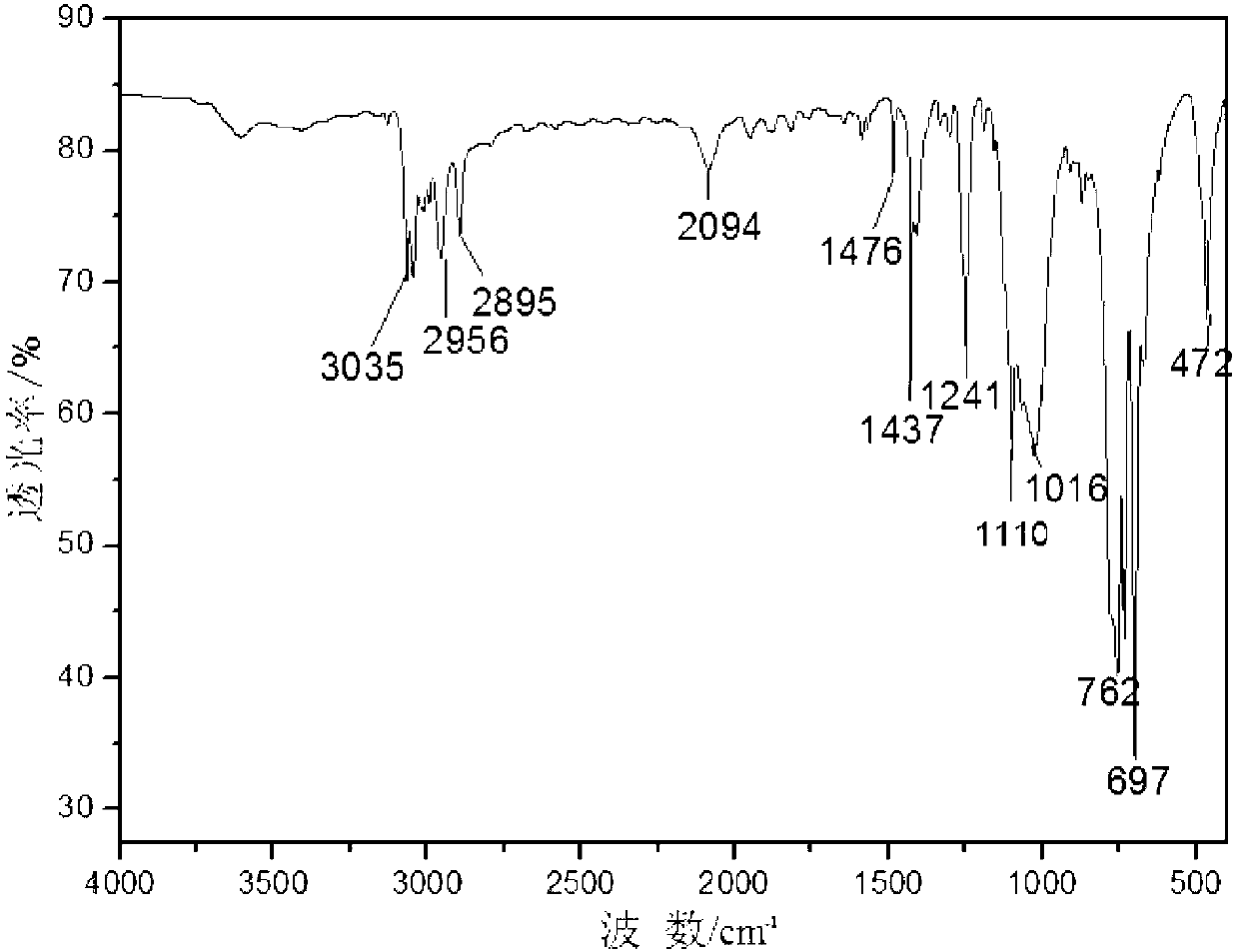
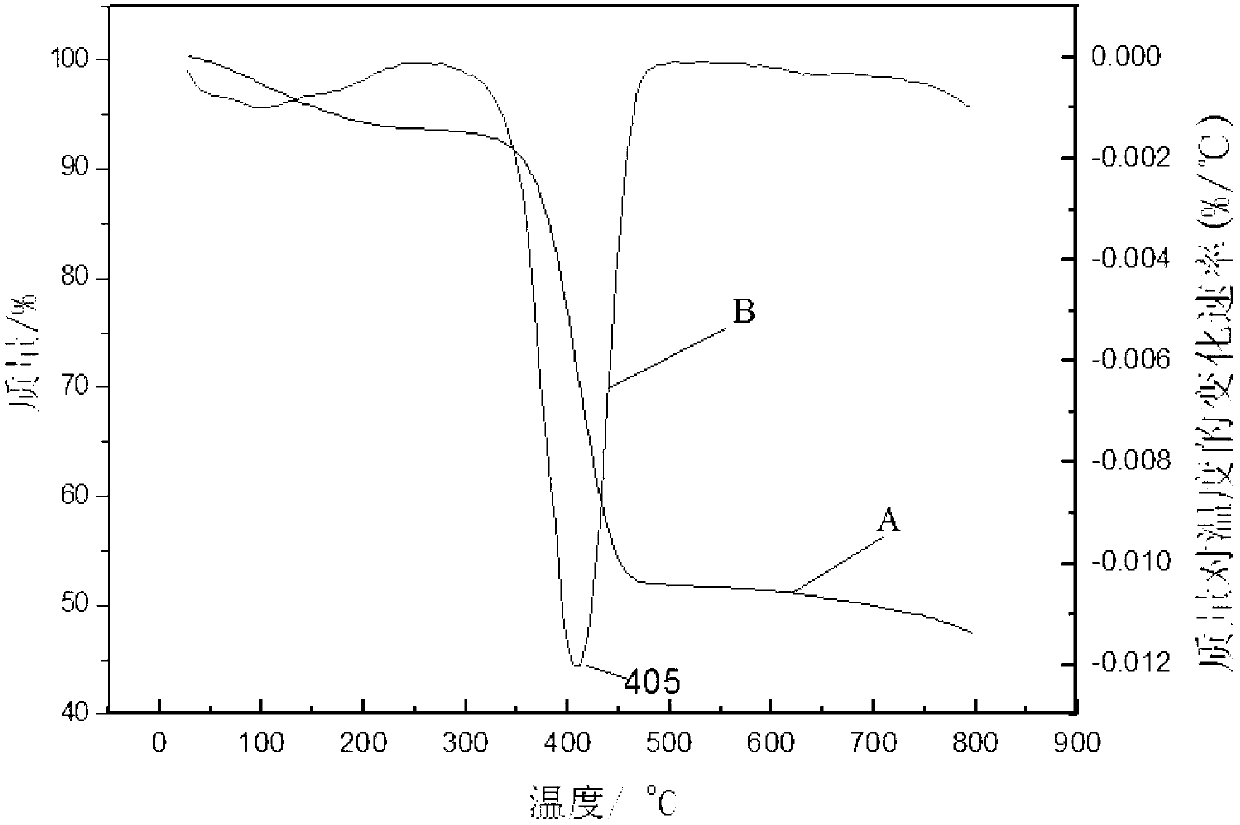
Polysilane azide and preparation method thereof
A technology for nitriding polysilane and polysilane, which is applied in the field of polysilane and its preparation, can solve the problems of less active groups, and achieve the effects of low viscosity, non-deformation, and wide application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0011] Embodiment 1: In this embodiment, polysilane azide is prepared from organic solvent, chlorine-terminated polysilane and sodium azide.
[0012] The quality of the chlorine-terminated polysilane described in this embodiment and the volume ratio of organic solvent is 1g:(3mL~10mL); The mass ratio of chlorine-terminated polysilane described in this embodiment and sodium azide is 1:(0.5 ~1).
[0013] The azide group is a high-energy group and a high-reactivity group, which can be used to obtain a series of heterocyclic compounds through cyclization with unsaturated bonds and hydrocarbons, and can be denitrified with trivalent phosphine organics at room temperature to obtain imidophosphine Derivatives; this embodiment successfully introduces azide groups into polysilane, which improves the situation that the practical application of polysilane is limited due to the existence of few active groups.
[0014] The existing chlorine-terminated polysilanes have the problems of vari...
specific Embodiment approach 2
[0015] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the organic solvent is N,N-dimethylformamide or dimethyl sulfoxide. Others are the same as the first embodiment.
specific Embodiment approach 3
[0016] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is: the chlorine-terminated polysilane is prepared from solvent, alkali metal and silane derivative / toluene solution; The silane derivative / toluene solution is prepared by mixing the silane derivative and toluene, and the concentration of the silane derivative in the silane derivative / toluene solution is 0.5g / mL~2g / mL; the mass of the alkali metal and the volume of the solvent The ratio is 1g:(2.5mL~15mL); the mass ratio of the alkali metal to the silane derivative / silane derivative in the toluene solution is 1:(2~9). Others are the same as those in Embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com