Recombined Tbeta 4-BP5 fusion peptide, gene, engineering bacteria and application
A technology of fusing peptides and engineering bacteria, applied in the field of bioengineering, can solve the problems of unsuitability for field promotion, high content of miscellaneous proteins, difficult purification, etc., to enhance the level of cellular immunity and humoral immunity of the body, broad application prospects, good immunity The effect of adjuvant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Design primers
[0048] The recombinant Tβ4-BP5 fusion peptide gene was designed according to the codon bias of Escherichia coli, as follows: 5'-ACTGACAAACCTGACATGGCCGAGATCGAGAAATTCGACAAATCGAAGCTCAAGAAGACTGAGACTCAAGAGAAGAATCCACTACCATCGAAGGAGACGATCGAGCAGGAGAAGCAAGCTGGCGAGTCCGGCGGCGGCGGCAGCTGCAAAAATGTGTAT-3' (SEQ ID NO: 1)
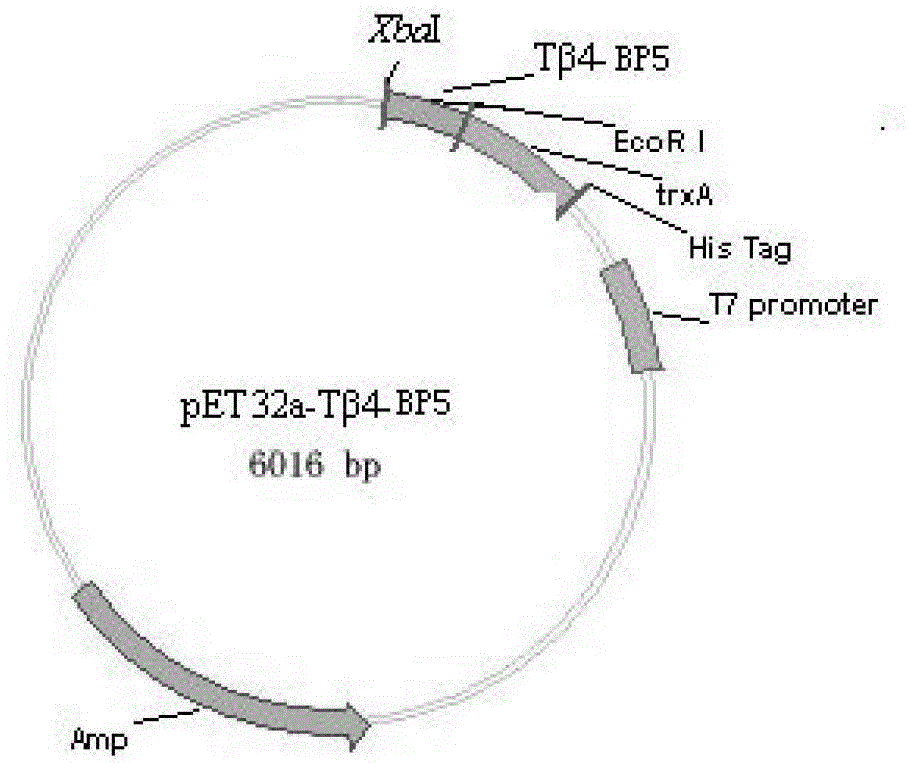
[0049] And design primer F for this fusion peptide gene 1 , F 2 and F 3 , where in primer F 1 Add EcoRI restriction site, primer F 3 Add stop codon and XbaI restriction site. The expression design diagram of the recombinant Tβ4-BP5 fusion peptide is shown in figure 1 , the primers were synthesized by Shanghai Invitrongen Company, as follows:
[0050] f 1 : 5'-CCG GAATTC ACTGACAAACCTGACATGGCCGAGATCGAGAAATTCGACAAATCGAAGCTCAAGAAGACTGA-3' (SEQ ID NO: 2), where the underlined part is the EcoRI restriction site;
[0051] f 2 : 5'-TCTCCTGCTCGATCGTCTCCTTCGATGGTAGTGGATTCTTCTCTTGAGTCTCAGTCTTCTTG-3' (SEQ ID NO: 3);
[0052] f 3 : 5'-TCGAGCAGGAGAA...
Embodiment 2
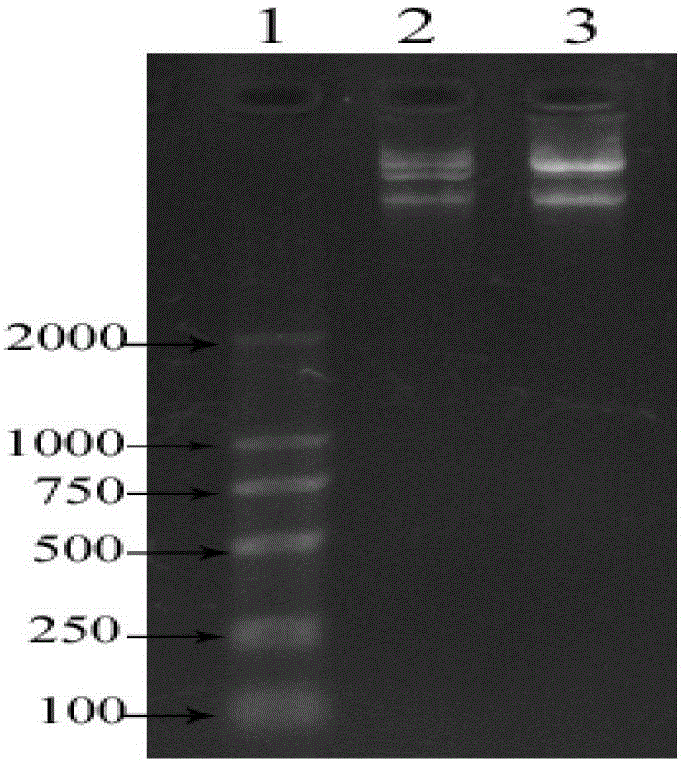
[0058] The recombinant Tβ4-BP5 fusion peptide gene of Example 1 and the plasmid vector pET32a were digested with EcoRI and XbaI, and placed in a 37°C water bath for 2 hours. After the digested products were identified by 1% agarose gel electrophoresis, the gel recovery reagent boxes for recovery identification. The digested recombinant Tβ4-BP5 fusion peptide gene and plasmid vector pET32a were ligated at a molar ratio of 1:3 overnight at 4°C. Take the ligation product and add it to a polypropylene centrifuge tube containing 100 μL of competent DH5α, mix gently and then ice-bath for 30 min. After the polypropylene centrifuge tube was taken out of the ice, it was heat-shocked at 42°C for 90 seconds, and then immediately ice-bathed for 2 minutes. Take it out and add it to 800 μL of LB medium preheated at 37°C, and shake (120rpm) at 37°C for 45min. Take 100 μL of the bacterial solution and spread it evenly on the agar LB plate containing 50 μg / mL of ampicillin (Amp), place it at...
Embodiment 3
[0061] The positive recombinant prokaryotic expression vector pET32a-Tβ4-BP5 obtained in Example 2 was transferred to a polypropylene centrifuge tube containing 200 μL of competent cells BL21, mixed gently and then ice-bathed for 30 minutes. Take it out and place it in a 42°C water bath, heat shock for 90s, and then immediately ice bath for 2min. Add 800 μL of 37°C preheated LB medium, and shake at 37°C for 45 minutes. Take 100 μL of the bacterial solution and evenly spread it on the agar LB plate (1 μg / mL ampicillin), place it upright at 37°C for 20 minutes, and incubate it upside down for 18 hours.
[0062] Pick a single colony from the agar LB plate (1 μg / mL ampicillin), inoculate it into 3 mL LB medium (1 μg / mL ampicillin), culture with shaking at 37 °C overnight, and transfer to 5 mL LB medium (1 μg / mL ampicillin) the next day ampicillin) at 37°C for 3 h with shaking, take 3 mL of the bacterial solution, and extract the plasmid according to the instructions of the rapid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com