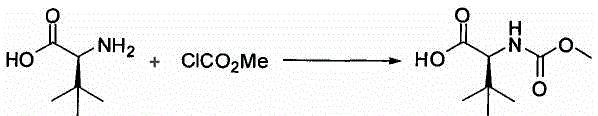A kind of preparation method of N-methoxycarbonyl-l-tert-leucine
A technology of tertiary leucine and tertiary leucine amine salt, which is applied in the field of medicine and chemical industry, can solve the problems of high cost, unsuitable large-scale industrial production of MOC-L-tertiary leucine, and difficulties in the separation and extraction of L-tertiary leucine and other problems, to achieve the effect of low cost, high product purity, simple and reasonable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add 765 g of ethyl acetate into a 1000 ml reaction flask, add 100 g of N-methoxycarbonyl-tert-leucine while stirring, and heat to 50-60°C. Stir to completely dissolve, add 66.7g of D-α-phenylethylamine, after the dropwise addition, keep warm for 10 minutes, cool down until the material precipitates, keep warm for 1 hour, then cool down to 0-5°C, keep warm for 1 hour, and filter with suction to get the wet product .
[0049] Add 240g of the above wet product into a 2000ml bottle, add 750g of water, stir to dissolve, and cool to 0-10°C. Add alkaline water dropwise to adjust the pH to about 11, extract with 1200ml of toluene, wash the toluene layer with 300g of water once, recover D-α-phenylethylamine from the toluene layer, pump 500g of ethyl acetate into the water layer, cool to 0-5°C, Use hydrochloric acid to adjust PH=1 to 3, control the temperature not to exceed 10°C, separate layers, add 100g of anhydrous magnesium sulfate to the organic layer and dry for 2 hours, f...
Embodiment 2
[0051] Add 765g of acetone into a 1000ml reaction flask, add 100g of N-methoxycarbonyl-tert-leucine while stirring, and heat to 40-45°C. Stir to completely dissolve, add 66.7kg of D-α-phenylethylamine, after the dropwise addition, keep warm for 10 minutes, cool until the material precipitates and keep warm for 1 hour, then cool down to -5°C to 0°C, keep warm for 1 hour, and filter with suction to get Wet product.
[0052] Add 120g of the above wet product into a 1000ml bottle, add 400g of water, stir to dissolve, and cool to 0-10°C. Add liquid alkali water dropwise to adjust the pH to about 11, extract with 600ml toluene, wash the toluene layer with 150g water once, recover D-α-phenylethylamine from the toluene layer, pump 300g ethyl acetate into the water layer, cool to 0-5°C, Use hydrochloric acid to adjust PH=1 to 3, control the temperature not to exceed 10°C, separate layers, add 50g of anhydrous magnesium sulfate to the organic layer and dry for 2 hours, filter with suct...
Embodiment 3
[0054] Add 600 g of isopropanol and 50 g of water into a 1000 ml reaction flask, add 100 g of N-methoxycarbonyl-tert-leucine while stirring, and heat to 45-50°C. Stir to completely dissolve, add 66.7kg of D-α-phenylethylamine, after the dropwise addition, keep warm for 10 minutes, cool until the material precipitates and keep warm for 1 hour, then cool down to -5°C to 0°C, keep warm for 1 hour, and filter with suction to get Wet product.
[0055] Add 60g of the above wet product into a 500ml bottle, add 200g of water, stir to dissolve, and cool to 0-10°C. Add liquid alkali water dropwise to adjust the pH to about 11, extract with 300ml toluene, wash the toluene layer with 80g water once, recover D-α-phenylethylamine from the toluene layer, pump 200g ethyl acetate into the water layer, cool to 0-5°C, Use hydrochloric acid to adjust PH=1 to 3, control the temperature not to exceed 10°C, separate layers, add 20g of anhydrous magnesium sulfate to the organic layer and dry for 2 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com