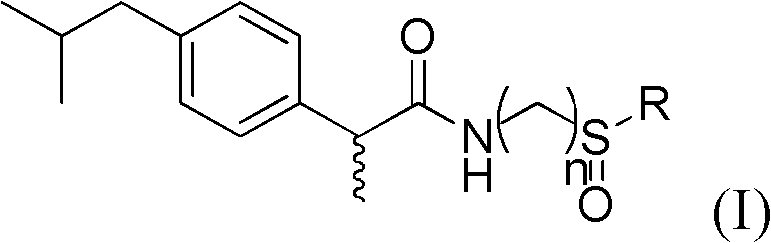
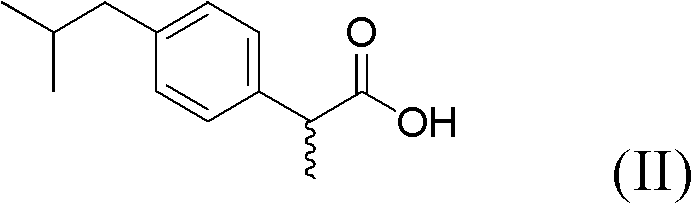
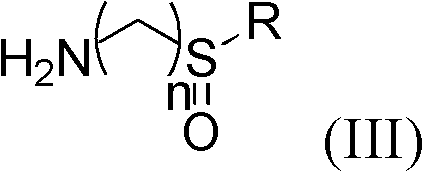Preparation and application of sulfonamide compound
A technology of sulfenamides and compounds, applied in the field of sulfenamide compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The starting materials used in the preparation of the compounds of the present invention are known, can be prepared according to known methods, or are commercially available.
[0043] The invention also relates to novel intermediates and / or starting materials. Particular preference is given to reaction conditions and novel intermediates which are the same or similar to those mentioned in the examples.
[0044] Both intermediates and final products can be worked up and / or purified according to conventional methods, including pH adjustment, extraction, filtration, drying, concentration, chromatography, trituration, crystallization, and the like.
[0045] In addition, the compounds of the present invention can be prepared by various methods known in the art or variations on the methods described herein.
[0046] The following examples are only used to illustrate the present invention and do not limit the present invention in any way.
Embodiment 1
[0047]The preparation of embodiment 1 (S)-N-((R)-tert-butylsulfinamide)-2-(4-isobutylphenyl) propanamide
[0048]
[0049] step 1
[0050] Take a 50ml round bottom flask, add CDI (0.41g, 2.52mmol) into (S)-2-(4-isobutylphenyl)propionic acid (0.52g, 2.52mmol) in a dry dichloromethane solution, The mixed solution was stirred at 0-5°C for 2 hours. Add (R)-(+)-tert-butylsulfinamide (0.31g, 2.52mmol) and DBU (0.3ml) and stir at room temperature for about 6 hours, follow the reaction by TLC, after the reaction is over. NaH for organic phase 2 PO 4 (2*5ml), washed with saturated saline (2*5ml). After drying over anhydrous sodium sulfate, the solvent was removed. Separation by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1) gave the target compound (0.62 g, yield 79%).
[0051] MS of the compound: [M+H] + 310.18; 1 H-NMR (400MHz CDCl 3 ): δδppm 7.16(q, 4H), 6.77(s, 1H CONH), 3.75(q, 1H), 2.46(d, 2H), 1.84(m, 1H), 1.56(d, 3H), 1.05(s , 9H), 0.89(d, ...
Embodiment 2
[0052] Preparation of Example 2 (R)-N-((R)-tert-butylsulfinamide)-2-(4-isobutylphenyl) propanamide
[0053]
[0054] step 1
[0055] Take a 50ml round bottom flask, add CDI (0.41g, 2.52mmol) into (R)-2-(4-isobutylphenyl)propionic acid (0.52g, 2.52mmol) in a dry dichloromethane solution, The mixed solution was stirred at 0-5°C for 2 hours. Add (R)-(+)-tert-butylsulfinamide (0.31g, 2.52mmol) and DBU (0.3ml) and stir at room temperature for about 6 hours, follow the reaction by TLC, after the reaction is over. NaH for organic phase 2 PO 4 (2*5ml), washed with saturated saline (2*5ml). After drying over anhydrous sodium sulfate, the solvent was removed. Separation by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1) gave the target compound (0.62 g, yield 79%).
[0056] MS of the compound: [M+H] + 310.18; 1 H-NMR (400MHz, CDCl 3 ): δδppm 7.16(q, 4H), 6.774(s, 1H CONH), 3.75(q, 1H), 2.46(d, 2H), 1.84(m, 1H), 1.56(d, 3H), 1.05(s , 9H), 0.89(d, 6H)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com