Six-element high-entropy alloy with first-order magnetic phase transition and preparation method thereof
A high-entropy alloy and magnetic phase transition technology, applied in the field of high-entropy alloys, can solve the problems that high-entropy alloys cannot achieve the first-order magnetic phase transition, and achieve critical transition temperature reversibility, large magnetic phase transition temperature range, and critical transition The effect of temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
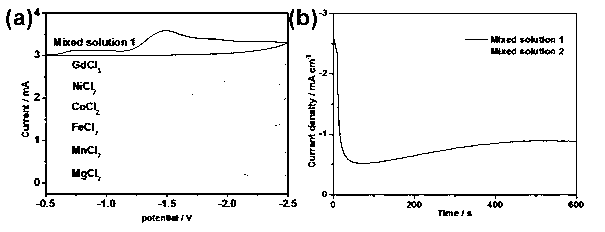
[0034] The composition of the electrolyte is as follows: DMSO, vitamin C (0.02 mol L ?1 ), (C 2 h 5 ) 4 NPF 6 (0.02 mol·L -1 ), GdCl 3 (0.015mol·L ?1 ), FeCl 2 (0.0075 mol L ?1 ), CoCl 2 (0.0075 mol L ?1 ), NiCl 2 (0.0075 mol L ?1 ), MnCl 2 (0.0075 mol L ?1 ) and MgCl 2 (0.015 mol L ?1 ). Using copper as the working electrode, platinum sheet as the counter electrode, and a saturated calomel electrode as the reference electrode, the electrochemical reduction deposition was carried out at 30°C, the deposition time was 1h, and the deposition potential was 2V.
[0035] By the above method, the composition is obtained as Mg 19.09 mn 7.83 Fe 29.09 co 16.93 Ni 17.91 Gd 9.15 alloy. Magnetic studies have shown (results such as Figure 4 shown), when the applied magnetic fields are 20, 40, 60 kOe, the transition temperatures are 210, 110, and 90K, respectively.
Embodiment 2
[0037] The composition of the electrolyte is as follows: DMSO, vitamin C (0.02 mol L ?1 ), (C 2 h 5 ) 4 NPF 6 (0.02 mol·L -1 ), GdCl 3 (0.02 mol·L ?1 ), FeCl 2 (0.01 mol·L ?1 ), CoCl 2 (0.01 mol·L ?1 ), NiCl 2 (0.01 mol·L ?1 ), MnCl 2 (0.01 mol·L ?1 ), MgCl 2 (0.02 mol·L ?1 ). Using copper as the working electrode, platinum sheet as the counter electrode, and a saturated calomel electrode as the reference electrode, the electrochemical reduction deposition was carried out at 30°C, the deposition time was 1h, and the deposition potential was 2V.
[0038] By the above method, the composition is obtained as Mg 12.03 mn 11.46 Fe 30.10 co 25.17 Ni 16.15 Gd 5.09 alloy. The magnetic results show that (such as Figure 5 As shown), the alloy undergoes a magnetic transition at about 60K when the applied magnetic field is 5000 Oe; and when the applied magnetic field is 10000 Oe, its transformation temperature drops to 30K.
Embodiment 3
[0040] The composition of the electrolyte is as follows: DMSO, vitamin C (0.02 mol L ?1 ), (C 2 h 5 ) 4 NPF 6 (0.02 mol·L -1 ), GdCl 3 (0.015 mol L ?1 ), FeCl 2 (0.01 mol·L ?1 ), CoCl 2 (0.01 mol·L ?1 ), NiCl 2 (0.01 mol·L ?1 ), MnCl 2 (0.01 mol·L ?1 ), MgCl 2 (0.015 mol L ?1 ). Using copper as the working electrode, platinum sheet as the counter electrode, and a saturated calomel electrode as the reference electrode, the electrochemical reduction deposition was carried out at 30°C, the deposition time was 1h, and the deposition potential was 2V.
[0041] By the above method, the composition is obtained as Mg 14.86 mn 6.02 Fe 34.74 co 15.66 Ni 22.99 Gd 5.73 alloy. The magnetic results show that (such as Figure 6 As shown), the alloy undergoes a magnetic transition at around 85K when the applied magnetic field is 5000 Oe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com