Method for synthesizing nitrogen-carbon nonmetal reducing catalyst by utilizing concentrated sulfuric acid carbonization
A carbon sulfate, chemical synthesis technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem of nitrogen active site loss, difficult to activate nitrogen, affecting oxygen reduction catalytic activity and other problems , to avoid nitrogen loss and improve the catalytic activity of oxygen reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
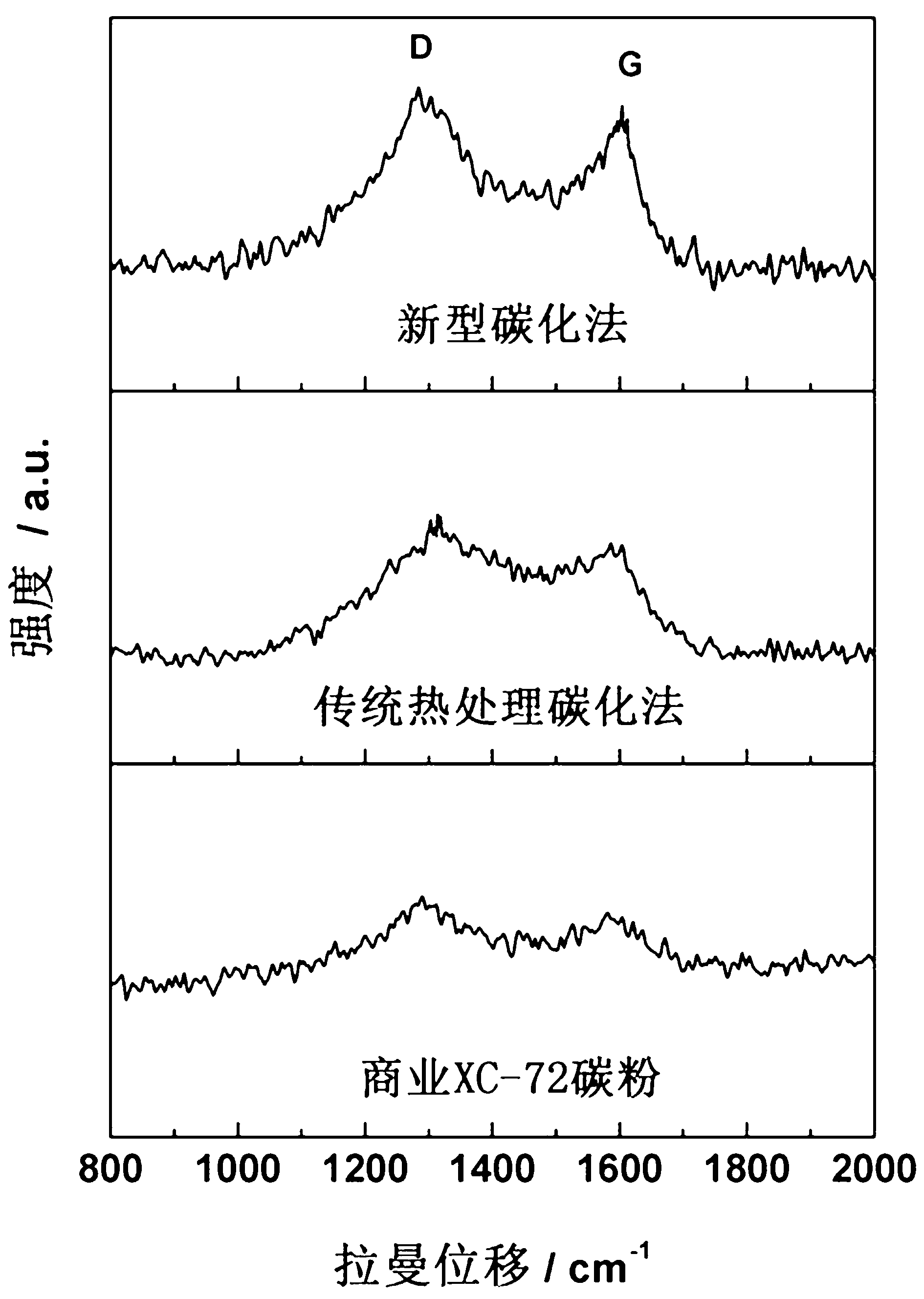
[0025] Add 4g sucrose and 2g melamine into a 400 mL beaker, mix and stir evenly, then add 20 mL of concentrated sulfuric acid with a mass concentration of 98% dropwise; then put it into an autoclave, and carbonize at 120°C for 10 h at a time; wait until the reaction is complete Then filter with suction, wash and dry. The obtained product was carbonized at a high temperature of 600 °C for 2 h under the protection of nitrogen to obtain a nitrogen-carbon composite non-metallic oxygen reduction catalyst material.
[0026] It is found by oxygen reduction test that on the electrode of the composite carbon material prepared by the present invention, the onset potential and the half-wave potential of the oxygen reduction reaction are respectively positively shifted by 49 mV and 30 mV compared with the catalyst prepared by the traditional heat treatment method; The XC-72 toner is positively shifted by 110 mV and 68 mV.
Embodiment 2
[0028] Add 4g of sucrose and 2g of melamine into a 400 mL beaker, mix and stir evenly, then add 20 mL of concentrated sulfuric acid with a mass concentration of 98% dropwise; Suction filtration, washing, and drying; the obtained product was subjected to secondary carbonization treatment at 800° C. for 2 h under the protection of nitrogen to obtain a non-metallic oxygen reduction catalyst material.
[0029] It is found by oxygen reduction test that on the electrode of the composite carbon material prepared by the present invention, the onset potential and the half-wave potential of the oxygen reduction reaction are respectively positively shifted by 59 mV and 36 mV compared with the catalyst prepared by the traditional heat treatment method; The XC-72 toner is positively shifted by 128 mV and 81 mV.
Embodiment 3
[0031] Add 4 g of inositol and 1 g of melamine into a 400 mL beaker, mix and stir evenly, then add 20 mL of concentrated sulfuric acid with a mass concentration of 98% dropwise, then put it into an autoclave, and carbonize at 120°C for 10 h at a time. After completion, suction filtration, washing, and drying; the obtained product was further carbonized at a high temperature of 600 °C for 2 h under the protection of nitrogen to obtain a non-metallic oxygen reduction catalyst material.
[0032] It is found by oxygen reduction test that on the electrode of the composite carbon material prepared by the present invention, the onset potential and the half-wave potential of the oxygen reduction reaction are respectively positively shifted by 42 mV and 29 mV compared with the catalyst prepared by the traditional heat treatment method; The XC-72 toner is positively shifted by 92 mV and 43 mV.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com