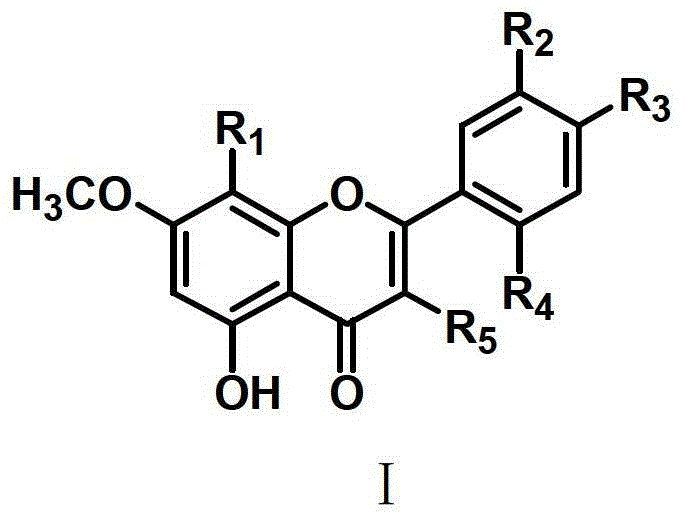
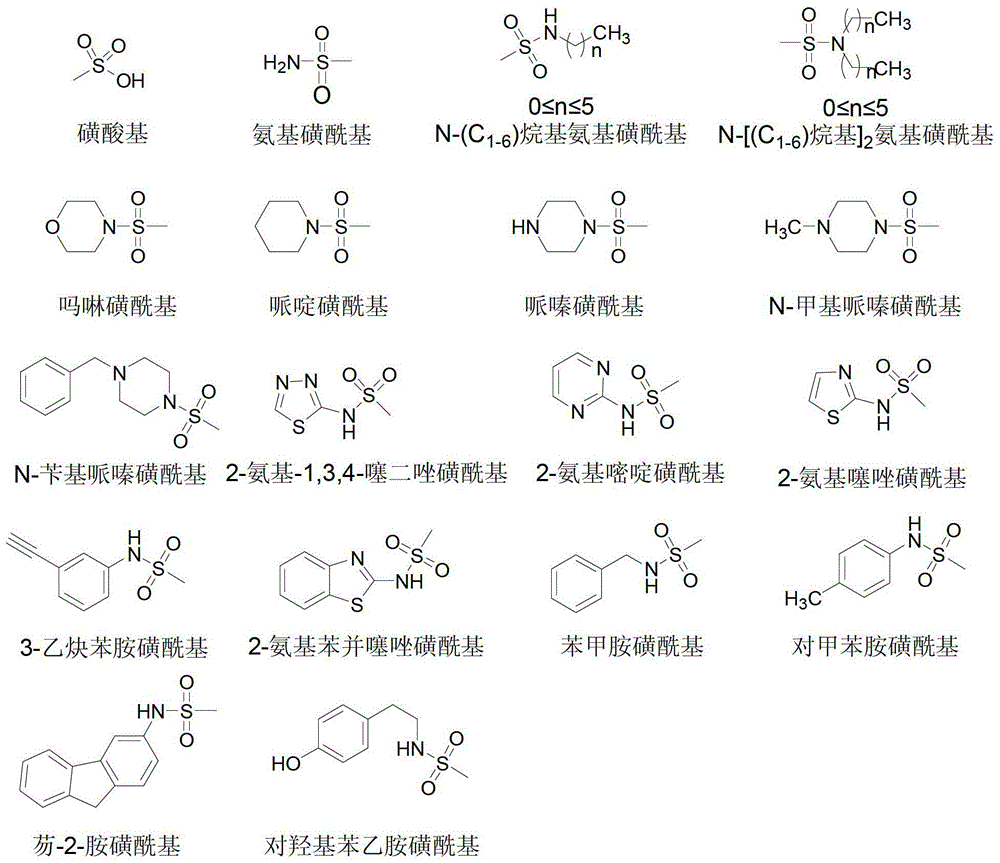
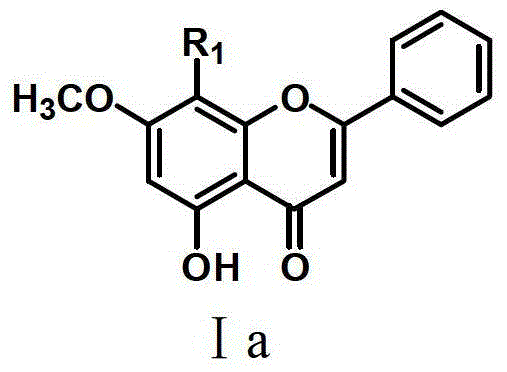
Flavone sulphonamide derivative with anti-cancer activity as well as preparation method and application thereof
A flavone sulfonamide and ketone sulfonamide technology, which is applied in the fields of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of no obvious tumor specificity and low anti-tumor activity in anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 18
[0052] Preparation of Example 18-p-Hydroxyphenethylsulfonyl-5-hydroxyl-7-methoxyflavone
[0053] (1) Weigh 0.508g (2mmol) of 5,7-dihydroxyflavone into a 250mL three-necked round-bottomed flask, add 100mL of acetone; at the same time weigh 0.552g of anhydrous potassium carbonate, dissolve it in 10mL of distilled water, and slowly add it to 5, In the acetone solution of 7-dihydroxyflavone, after stirring at room temperature for half an hour, add 148 μL iodomethane, react at 40°C for 8 hours, concentrate the reaction solution under reduced pressure, wash with distilled water, and obtain 0.520 g of light yellow 5-hydroxy-7-methoxy flavonoids. (2) Take 13.2 mL of chlorosulfonic acid in a three-necked flask, cool to -5°C, add 2 mmol of 5-hydroxy-7-methoxyflavone in batches and stir for 3 hours. After the reaction is completed, pour the reaction solution into ice water in batches , extracted 3 times with ethyl acetate, combined the ethyl acetate extracts, concentrated and dried unde...
Embodiment 28
[0054] Preparation of Example 28-Benzylaminosulfonyl-5-hydroxyl-7-methoxyflavone
[0055] The synthesis method was the same as in Example 1, wherein the p-hydroxyphenethylamine in step (3) was replaced by benzylamine (1.1 mmol), and the target compound was synthesized as a light brown solid powder with a yield of 40.7%. The results of instrumental analysis of the compound structure: 1 H-NMR (CDCl 3 ,400MHz)δ13.91(1H,s,5-OH),8.21(2H,d,J=8.0Hz,H-2′,H-6′),7.56(3H,m,H-3′,H -4′,H-5′),7.13-7.19(5H,m,ArH),6.82(1H,s,H-3),6.32(1H,s,H-6),5.46(1H,t,J =6.0Hz,NH),4.24(2H,d,J=6.0Hz,CH 2 ),3.90(3H,s,CH 3 O). 13 C-NMR (CDCl 3 ,100MHz) δ182.4, 166.1, 165.3, 162.6, 155.3, 135.9, 132.5, 130.3, 129.3, 128.5, 128.0, 127.9, 127.2, 107.6, 105.4, 105.1, 95.9, 57.2, 48.1. MS m / z[M+H] + : 438.25.
Embodiment 38
[0056] Example 38 - Preparation of p-methylanilinesulfonyl-5-hydroxyl-7-methoxyflavone
[0057] The synthesis method is the same as in Example 1, wherein p-hydroxyphenethylamine in step (3) is replaced by p-methylaniline (1.1 mmol), and the target compound is synthesized as light yellow solid powder with a yield of 61.3%. The results of instrumental analysis of the compound structure: 1 H-NMR (CDCl 3 ,400MHz)δ13.94(1H,s,5-OH),8.18(2H,dd,J=7.6,1.6Hz,H-2′,H-6′),7.52-7.58(3H,m,H- 3′,H-4′,H-5′),7.11(1H,s,NH),6.99(2H,dd,J=6.0,2.8Hz,H-3′′,H-5′′),6.98 (2H,dd,J=6.0,2.8Hz,H-2'',H-6''),6.77(1H,s,H-3),6.42(1H,s,H-6),4.11(3H ,s,CH 3 O),2.21(3H,s,CH 3 ). 13 C-NMR (CDCl 3 ,100MHz) δ182.4, 166.5, 165.3, 163.0, 156.1, 135.4, 134.0, 132.5, 130.3, 130.0, 129.3, 127.2, 121.2, 106.0, 105.4, 105.2, 95.9, 57.4, 20.8. MS m / z[M+H] + : 438.25.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com