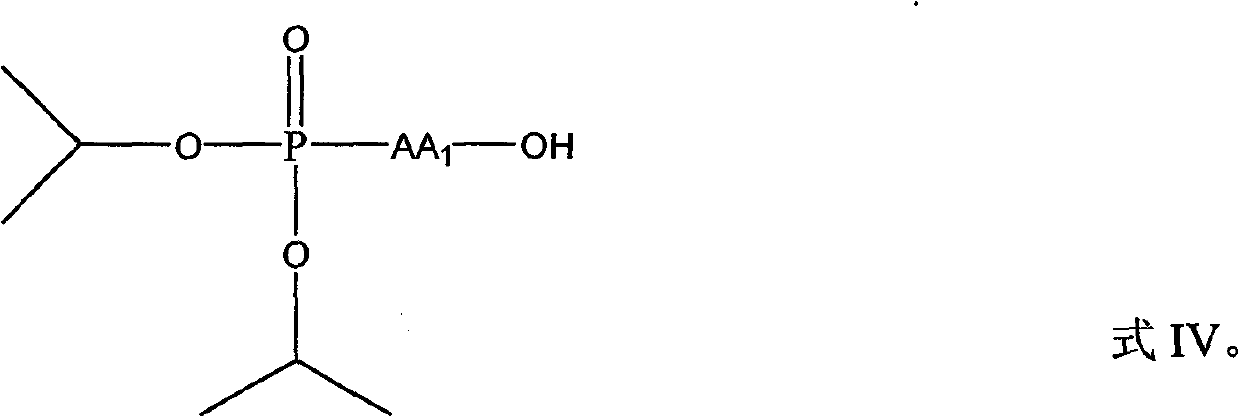Phosphorylated amino acid stilbene derivative and its preparation method and application
An aminostilbene, phosphorylated amino acid technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as urgent research, strong side effects and drug resistance, To achieve the effect of simple reaction, inhibition of tumor activity, and correct structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
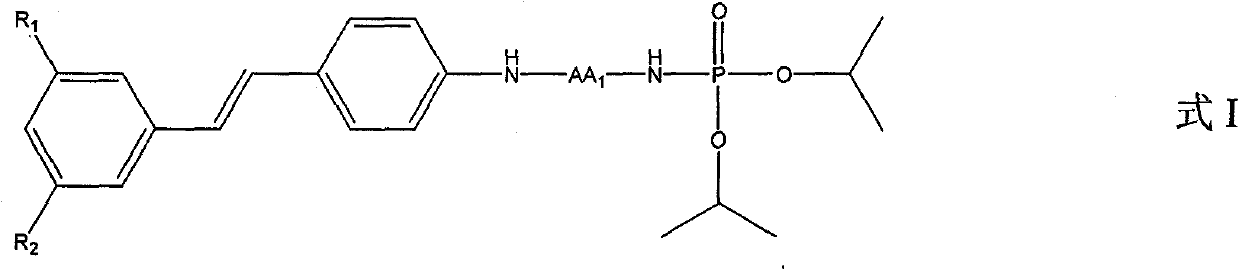
[0019] Example 1. Synthesis of trans 3,5-dimethoxy-4'-N-diisopropoxyphosphorylated amino acid stilbene compound
[0020] Add 2mmol 3,5-dimethoxy-4'-aminostilbene and 10mL dry tetrahydrofuran into a 50mL flask, add 2.1mmol N-phosphorylated amino acid (diisopropoxyphosphorylated amino acid) and 2.1mmol·L -1 HOBt (1-hydroxybenzotriazole) solution, add dropwise a condensing agent 2.1mmol DCC (N,N'-dicyclohexylcarbodiimide) and 5mL dry tetrahydrofuran mixed solution in an ice-water bath, and react overnight at room temperature Filtration, the residue was washed three times with THF (tetrahydrofuran), the filtrate and washings were combined, the solvent was removed by rotary evaporation, and the residue was saturated with NaHCO 3 solution, fully stirred and extracted with chloroform, the combined extracts were sequentially washed with saturated NaHCO 3 solution, citric acid aqueous solution with a mass fraction of 10%, and saturated NaCl solution were washed three times, and anhyd...
Embodiment 2
[0033] Example 2, Synthesis of trans 4-N-diisopropoxyphosphorylated amino acid stilbene compound
[0034] Add 2mmol trans-4-aminostilbene and 10mL dry tetrahydrofuran into a 50mL flask, add 2.1mmol N-phosphorylated amino acid and 2.1mmol·L -1 HOBt (1-hydroxybenzotriazole), dropwise add a condensing agent 2.1mmol DCC (N,N'-dicyclohexylcarbodiimide) and 5mL dry tetrahydrofuran mixed solution in an ice-water bath, react overnight at room temperature and filter , the residue was washed three times with tetrahydrofuran, the filtrate and washings were combined, the solvent was removed by rotary evaporation, and the residue was added with saturated NaHCO 3 solution, fully stirred and extracted with chloroform, and the combined extracts were sequentially washed with saturated NaHCO 3 solution, citric acid solution with a mass fraction of 10%, and saturated NaCl solution were washed three times, dried over anhydrous Na2SO4, filtered, washed, and rotary evaporated to remove chloroform,...
Embodiment 3
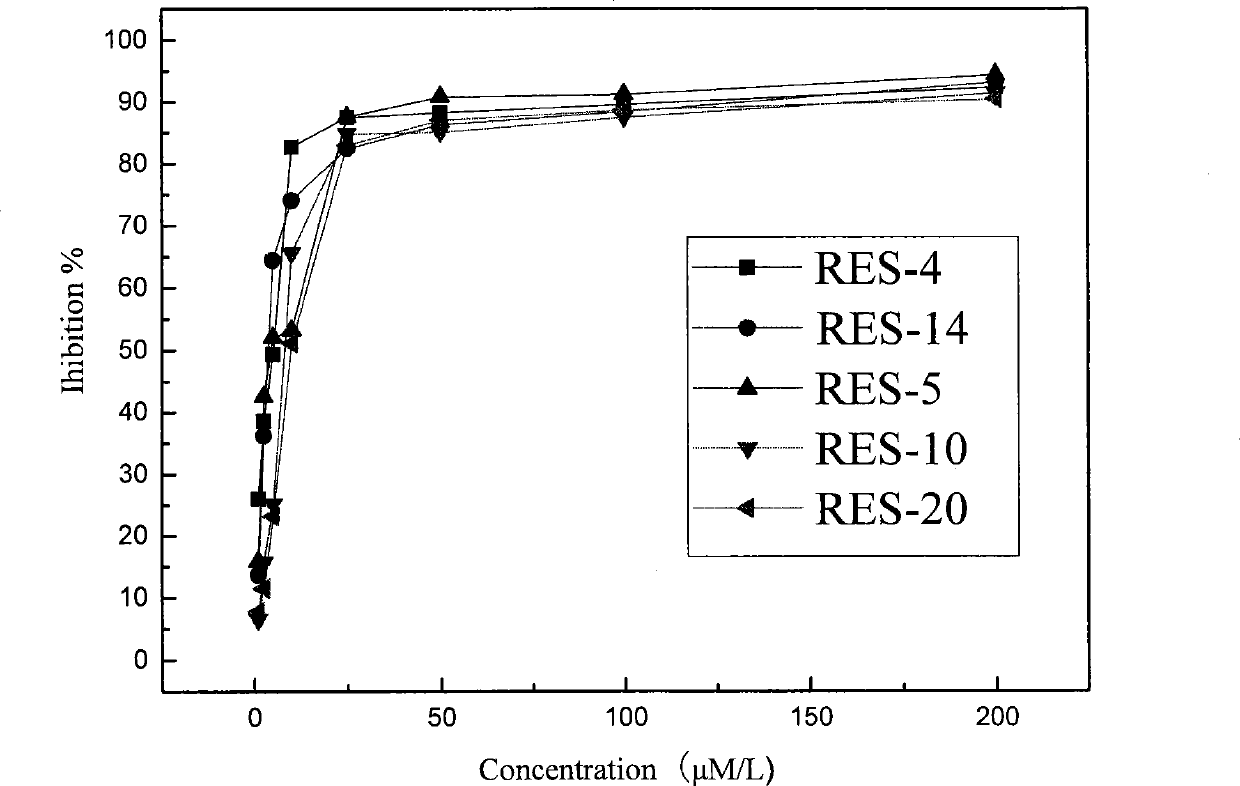
[0050] Example 3, MTT method cell proliferation inhibitory activity screening
[0051] Take the CNE1 and CNE2 cells (two human nasopharyngeal carcinoma cell lines) in the logarithmic growth phase, and divide them into 2×10 5 Inoculate in a 96-well plate at a density of 1 / mL, 99 μL / well, at 37°C, 5% CO 2 After culturing in the incubator for 4 hours, the compounds prepared in Examples 1 and 2 of the present invention were added to each well so that the final concentrations were 200 μmol / L, 100 μmol / L, 50 μmol / L, 25 μmol / L, 10 μmol / L, 5 μmol / L, 2.5μmol / L, 8 concentration gradients of 1μmol / L. Three replicate wells were set up for each compound, and both negative and positive controls were set up, wherein, the negative control was 1% methanol solution by volume, and the positive control was resveratrol. After acting for 72 hours, add MTT (5mg / ml) solution, 10μL / well, continue to cultivate for 4-6 hours, centrifuge at 2000rpm, 4°C for 5 minutes, absorb the supernatant, add DMSO,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com