In vivo copper-free click chemistry for delivery of therapeutic and/or diagnostic agents
A technology of click chemical reaction and therapeutic agent, applied in the field of 18F or 19F labeled peptide or other molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Example 1. Peptide IMP272 18 F mark
[0193] prepare and use 18The first peptide labeled with F is IMP272: DTPA-Gln-Ala-Lys(HSG)-D-Tyr-Lys(HSG)-NH 2 (SEQ ID NO: 3).
[0194] Acetate buffer solution - 1.509 g of acetic acid was diluted in ~160 mL of water and the pH was adjusted by adding 1M NaOH, then diluted to 250 mL to make a 0.1 M solution at pH 4.03.
[0195] Aluminum acetate buffer solution - by adding 0.1028g of AlCl 3 The aluminum solution was prepared by diluting the hexahydrate in 42.6 mL DI water. A 4 mL aliquot of the aluminum solution was mixed with 16 mL of a pH 4 0.1 M NaOAc solution to provide a 2 mM Al stock solution.
[0196] IMP272 acetate buffer solution-peptide, 0.0011g, 7.28×10 -7 mol IMP272 was dissolved in 364 μL of 0.1 M pH 4 acetate buffer solution to obtain a 2 mM peptide stock solution.
[0197] F-18 Labeling of IMP272 - Place 3 µL aliquots of aluminum stock solution in REACTI-VIAL TM medium and with 50μL 18 F (as received) was mixed...
Embodiment 2
[0198] Example 2. Peptide IMP272 with other metals 18 F mark
[0199] Aliquot 3 μL metal stock solution (6 × 10 -9 mol) was placed in a polypropylene conical flask and mixed with 75 μL 18 F (as received) was mixed, incubated at room temperature for ~2min, and then mixed with 20μL of 2mM (4×10 -8 mol) IMP272 solution mixed. The solution was heated in a heating block at 100°C for 15 min and analyzed by reverse phase HPLC. IMP272 (not shown) was labeled with indium (24%), gallium (36%), zirconium (15%), lutetium (37%) and yttrium (2%). These results demonstrate that, 18 F metal labeling techniques are not limited to aluminum ligands, but other metals can be utilized as well. Depending on the metal ligand, different chelating moieties can be utilized to optimize binding of the F-18-metal conjugate.
Embodiment 3
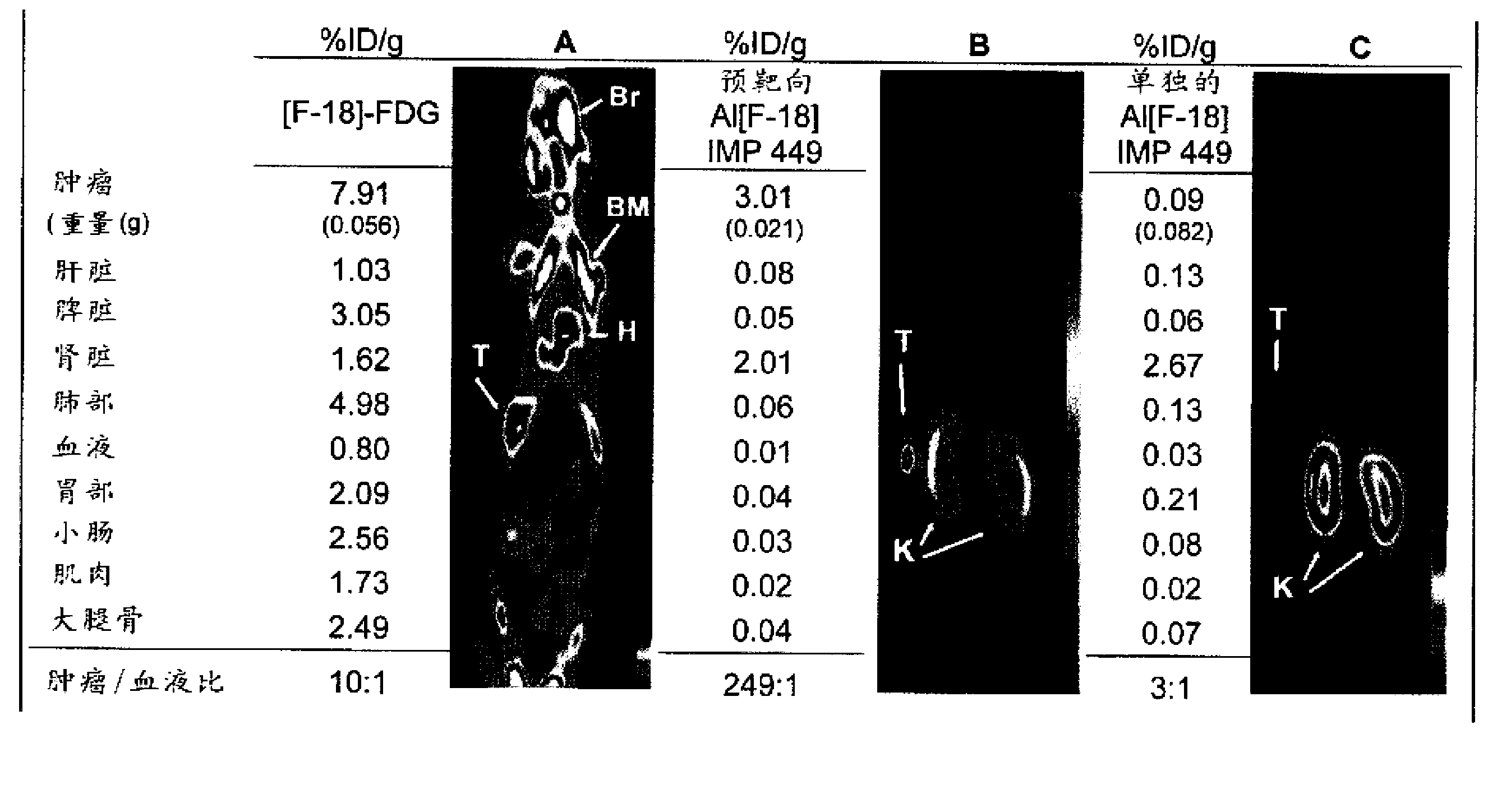
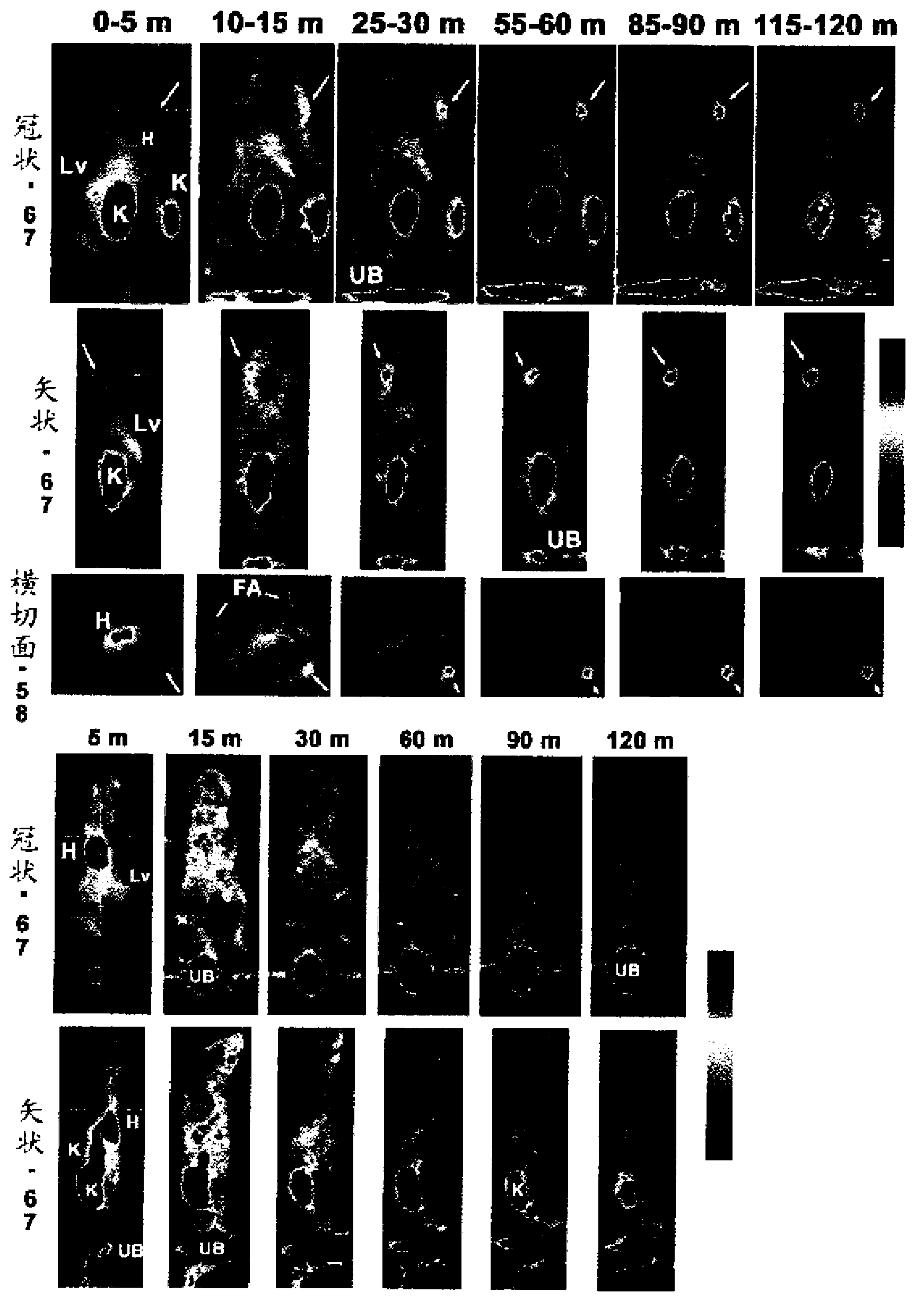
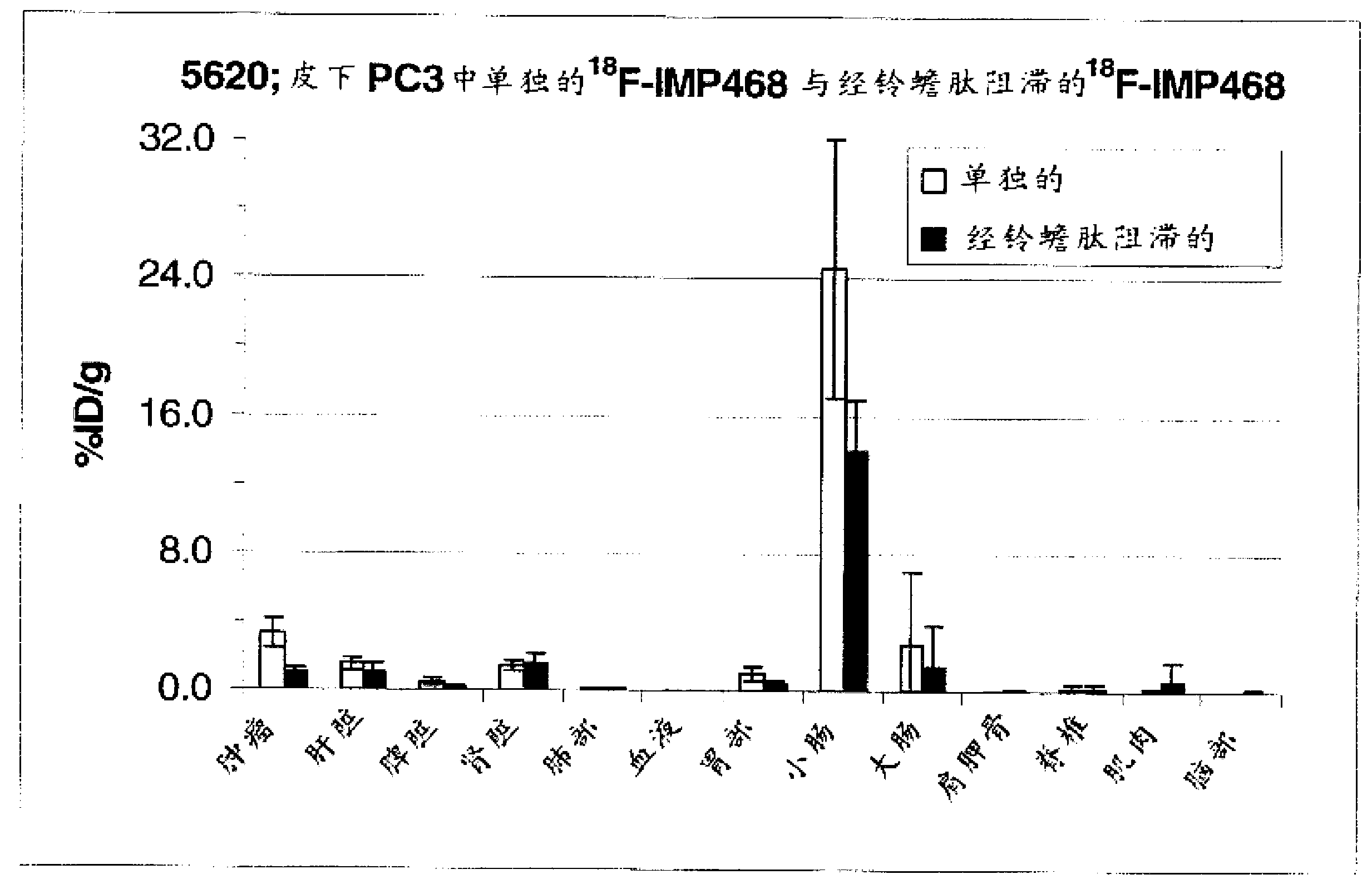
[0200] Example 3. Serum stable 18 Generation and use of the F-labeled peptide IMP449
[0201] IMP449
[0202] NOTA-ITCBenzyl-D-Ala-D-Lys(HSG)-D-Tyr-D-Lys(HSG)-NH 2 (SEQ ID NO:4)
[0203] On Sieber amide resin, peptide IMP448D-Ala-D-Lys(HSG)-D-Tyr-D-Lys(HSG)-NH was prepared by adding the following amino acids to the resin in the order indicated 2 (SEQID NO:5): Aloc-D-Lys(Fmoc)-OH, Trt-HSG-OH, cut Aloc, Fmoc-D-Tyr(But)-OH, Aloc-D-Lys(Fmoc)-OH, Trt -HSG-OH, cleaves Aloc, Fmoc-D-Ala-OH, and finally Fmoc to make the desired peptide. The peptide was then cleaved from the resin and purified by HPLC to generate IMP448, which was then coupled to ITC-benzyl NOTA.
[0204] Make IMP448 (0.0757g, 7.5×10 -5 mol) and 0.0509g (9.09×10 -5 mol) ITC benzyl NOTA was mixed and dissolved in 1 mL of water. Anhydrous potassium carbonate (0.2171 g) was then added slowly to the stirred peptide / NOTA solution. The pH of the reaction solution after all the carbonate addition was 10.6. The reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com