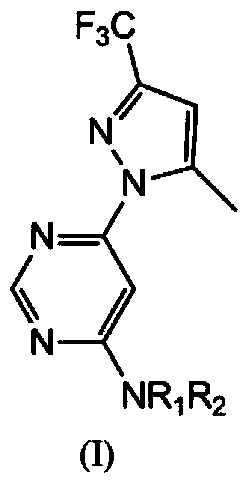
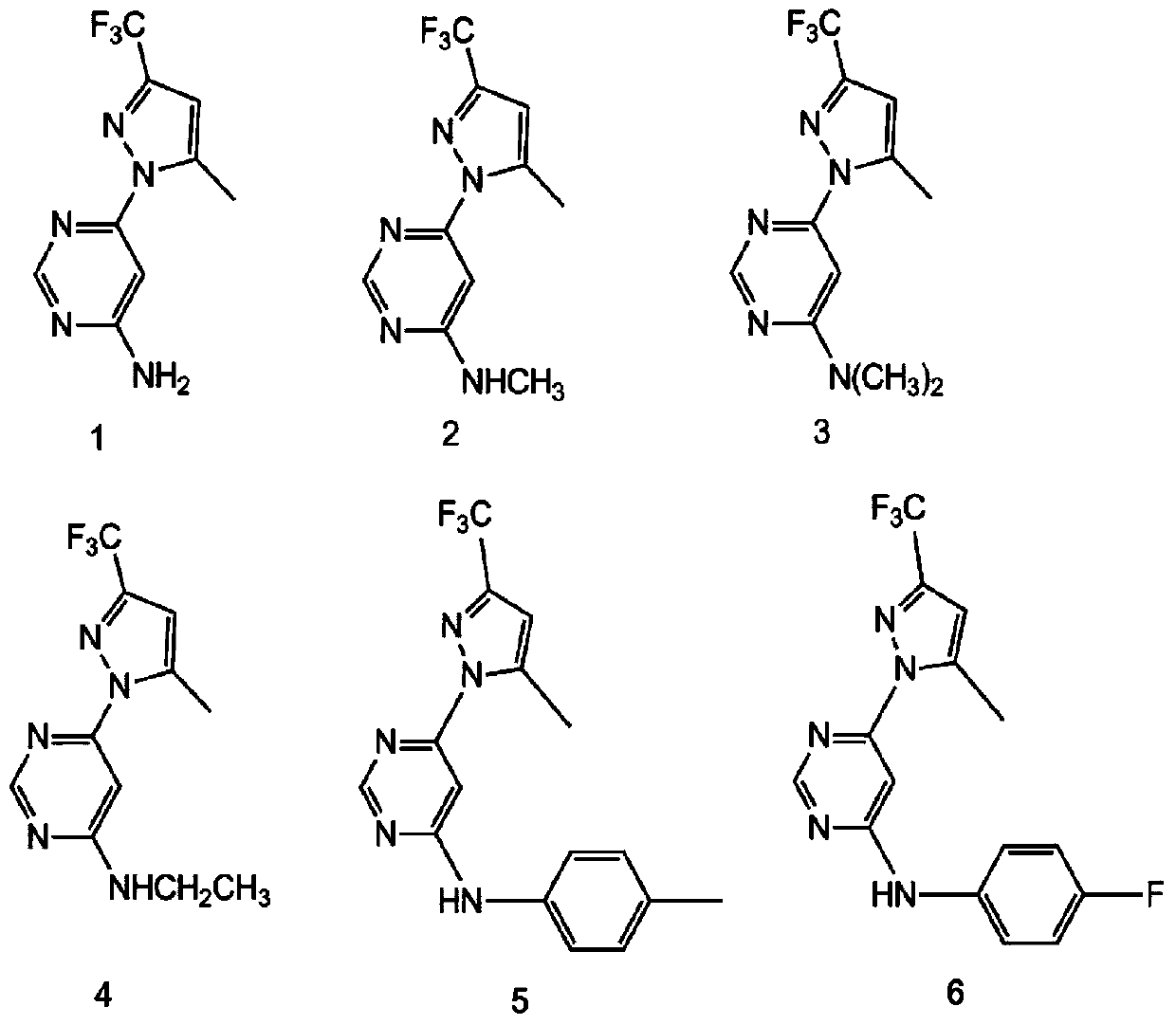
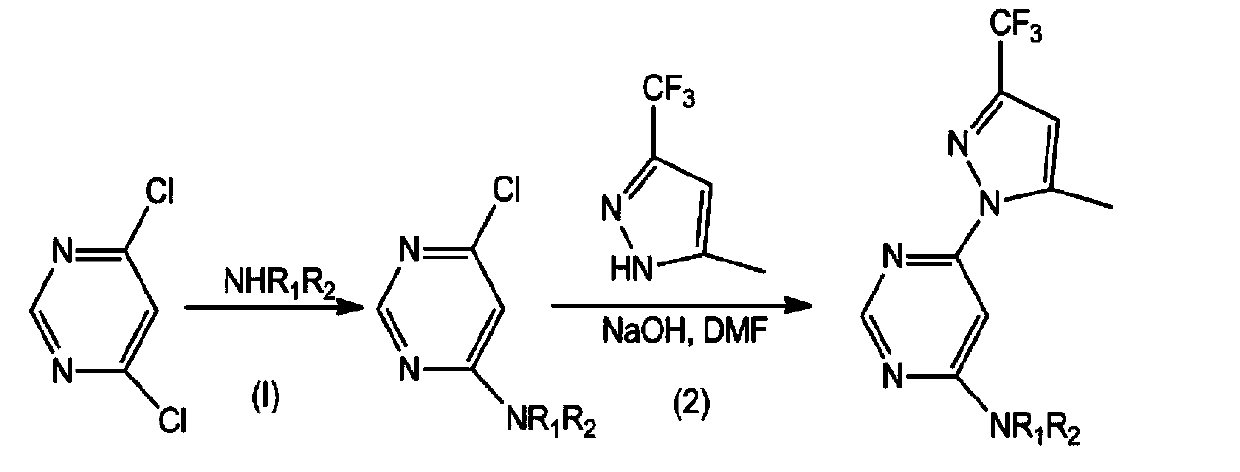
Pyrilamine compounds with herbicidal activity and application thereof
A technology of pyrimidine amines and compounds, which is applied in the field of pyrimidine amines and can solve problems such as herbicides having no research effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of intermediate 3-trifluoromethyl-5 methylpyrazole
[0022]
[0023] Add 1.57g (10mmol, 98%) of 1,1,1-trifluoro-2,4-pentanedione and 0.42g (10.5mmol, 80%) of hydrazine hydrate into 60mL of ethanol, reflux for 6h, and stop the reaction , concentrated under reduced pressure to leave about 10mL of solvent, left to stand, crystallized, and the solid was recrystallized with 95% ethanol to obtain the target compound. Dry to obtain 1.20 g of white solid, yield 80%, melting point 88-90 ° C, IR (KBr, cm -1 )ν: 3256(NH), 2943(CH 3 ), 1594 and 1485 (pyrazolyl); 1 H NMR (CDCl 3 ,300MHz):2.35(s,3H,CH 3 ), 6.30 (s, 1H, pyrazole-4H).
Embodiment 2
[0025] Intermediate 4-amino-6-chloropyrimidine preparation of
[0026] Add 2.98g (20mmol) of 4,6-dichloropyrimidine and 10mL DMF to a 100mL two-necked bottle, stir to dissolve, then add 40mL concentrated ammonia water to react at 30°C, follow the reaction process by TLC, and stop the reaction when no more products are formed. Then add 40mL of ice water, let it stand still, a large amount of crystals are precipitated, filter, wash with water, and dry to obtain 2.04g of milky white solid product 4-amino-6-chloropyrimidine, melting point 208-210°C, yield 78.8%, IR (KBr tablet, cm -1 ): 3313 (NH 2 ), 3134 (NH 2 ), 1665, 1583, 1528, 1410, 1347, 1259, 1086, 989, 923, 752. Infrared data indicated that the amino group was attached.
Embodiment 3
[0027] Embodiment 3: Intermediate 6-chloro-4-methylaminopyrimidine preparation of
[0028] Add 2.98g (20mmol) of 4,6-dichloropyrimidine and 40mL of ethanol to a 100mL two-necked bottle with a reflux condenser and a tail gas absorption device, stir to dissolve, then add 1.62g (24mmol) of methylamine hydrochloride and 3.35 mL (24mmol) triethylamine, heated to reflux, when TLC monitors that no more products are produced, stop the reaction, cool, pour the reaction solution into a 100mL round bottom flask, desolvate under reduced pressure, a large amount of white solid appears, add a small amount of water, Filtered, washed 3 times with a small amount of water, suction filtered, and dried to obtain 2.13 g of white solid product 6-chloro-4-methylaminopyrimidine, melting point: 136-138 ° C, yield 74.2%, IR (KBr tablet, cm -1 ): 3264 (b, NH), 2930, 1601, 1130, 972, 848.
[0029]
[0030] Intermediates 6-chloro-4-N, N-dimethyl-pyrimidinyl-4-amine, 6-chloro-N-ethyl-pyrimidinyl-4-am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com