Industrialized gemcitabine hydrochloride synthesis method
A gemcitabine hydrochloride, synthesis technology, applied in the field of gemcitabine hydrochloride synthesis technology, can solve the problem of incomplete reaction, affecting product quality and yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
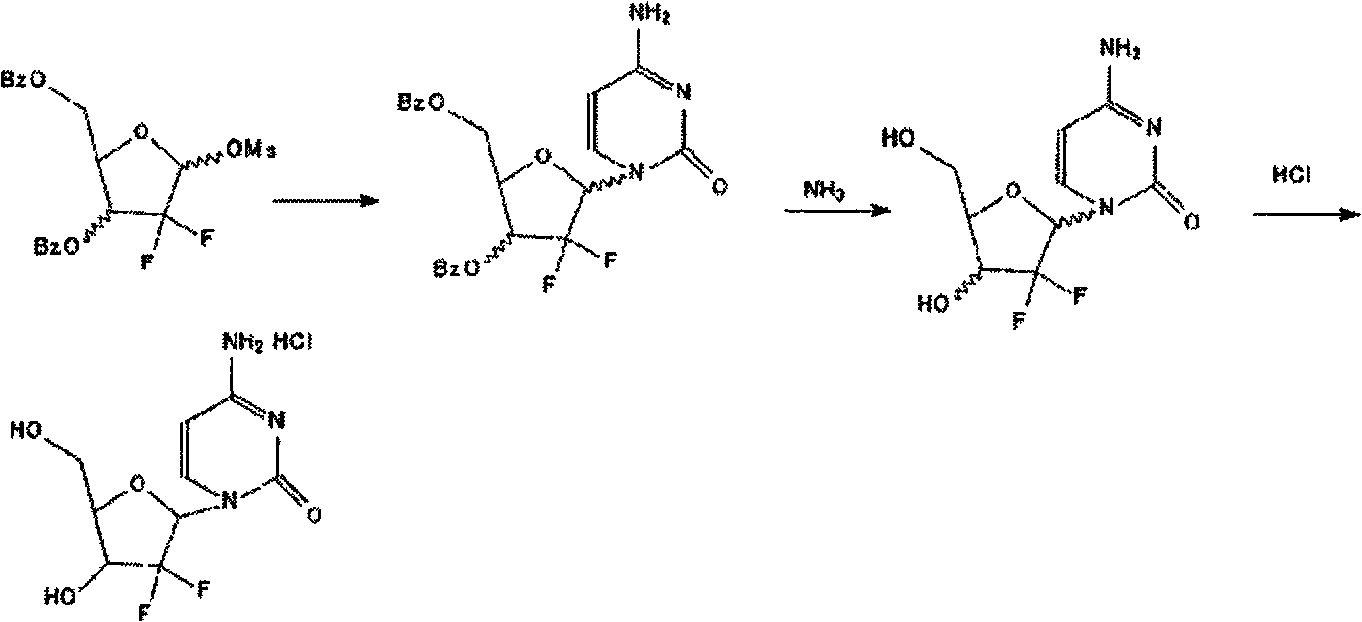
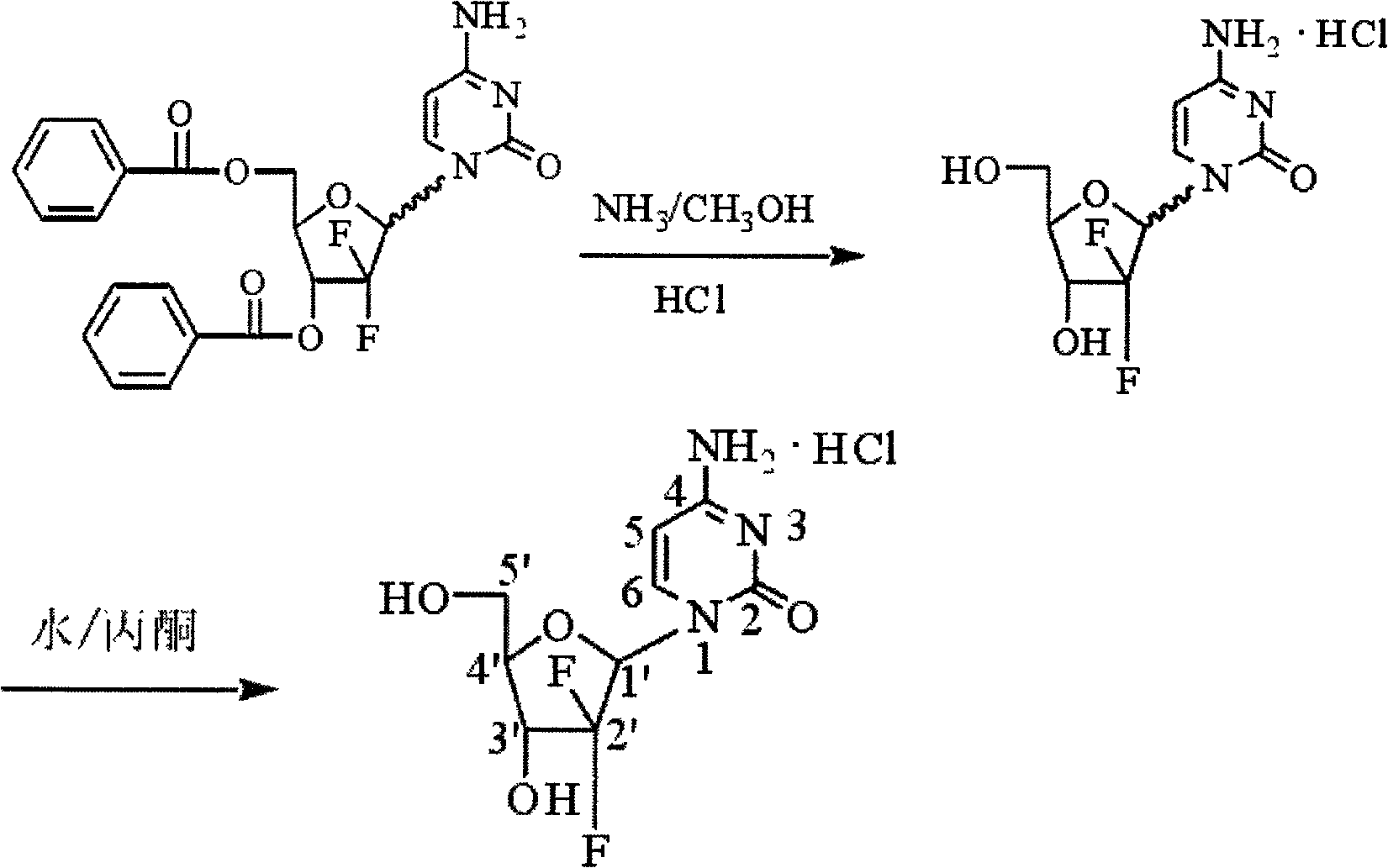
[0019] 2'-deoxy-2', 2'-difluorocytidine-3', 5'-dibenzoate (10g) in methanol (325ml) was cooled to 0°C, 10ml of concentrated ammonia was added, and the reaction was stirred overnight at room temperature , concentrated under reduced pressure, the remaining oil was dissolved in water (100ml), washed with ethyl acetate (100ml×2), the organic layers were combined and extracted with water (100ml), the aqueous layers were combined, decolorized with activated carbon, filtered, and the filtrate was concentrated under reduced pressure to After drying, isopropanol (100ml) and concentrated hydrochloric acid (13ml) were added to the residue, heated to 70°C, kept at room temperature for 0.5h and left to stand overnight. Filtration, the filter cake was washed successively with cold isopropanol (13ml) and n-hexane (6ml), and dried to obtain a white solid, 2'-deoxy-2', 2'-difluorocytidine hydrochloride 4.2g, yield 65%.
[0020] Put 2'-deoxy-2', 2'-difluorocytidine hydrochloride (4.1g) and wat...
Embodiment 2
[0022] 2'-deoxy-2', 2'-difluorocytidine-3', 5'-dibenzoate (20g) in methanol (650ml) was cooled to 0°C, 20ml of concentrated ammonia was added, and the reaction was stirred at room temperature overnight , concentrated under reduced pressure, the remaining oil was dissolved in water (200ml), washed with ethyl acetate (200ml×2), the organic layers were combined and extracted with water (200ml), the aqueous layers were combined, decolorized with activated carbon, filtered, and the filtrate was concentrated under reduced pressure to After drying, isopropanol (200ml) and concentrated hydrochloric acid (26ml) were added to the residue, heated to 70°C, kept at room temperature for 0.5h and left to stand overnight. Filtration, the filter cake was washed successively with cold isopropanol (25ml) and n-hexane (12ml), and dried to obtain a white solid, 2'-deoxy-2', 2'-difluorocytidine hydrochloride 8.1g, yield was 64%.
[0023] Put 2'-deoxy-2', 2'-difluorocytidine hydrochloride (8.1g) an...
Embodiment 3
[0025] 2'-deoxy-2', 2'-difluorocytidine-3', 5'-dibenzoate (40g) in methanol (1300ml) was cooled to 0°C, 40ml of concentrated ammonia was added, and the reaction was stirred overnight at room temperature , concentrated under reduced pressure, the remaining oil was dissolved in water (400ml), washed with ethyl acetate (400ml×2), the organic layers were combined and extracted with water (400ml), the aqueous layers were combined, decolorized with activated carbon, filtered, and the filtrate was concentrated under reduced pressure to After drying, isopropanol (400ml) and concentrated hydrochloric acid (52ml) were added to the residue, heated to 70°C, kept at room temperature for 0.5h and left to stand overnight. Filtration, the filter cake was washed successively with cold isopropanol (50ml) and n-hexane (24ml), and dried to obtain a white solid, 2'-deoxy-2', 2'-difluorocytidine hydrochloride 16g, the yield was 61%.
[0026] Put 2'-deoxy-2', 2'-difluorocytidine hydrochloride (16g)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com