Application of bixanthone compound FLBG-1108 or its pharmaceutical salts in preparation of anti-cancer drugs
A technology of ketone compounds and anticancer drugs, which is applied to the application field of bixanthone compounds in the preparation of anti-lung cancer drugs, can solve the problems of rarity, toxicity, side effects, and gastrointestinal discomfort of patients, and achieve anti-cancer drugs. Clear cancer effect, less toxic and side effects, strong selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Antitumor activity of bixanthone compound FLBG-1108
[0030] The present invention adopts an in vitro cytotoxicity test to measure the antitumor activity of the compound FLBG-1108 by detecting the survival rate of human cancer cells cultured in vitro after being treated with different concentrations of the FLBG-1108 for 48 hours.
[0031] The selected tumor cell lines are: human non-small cell lung cancer H520, A549, H549, 1299, human liver cancer cell HepG2, human breast cancer cell MCF7, human prostate cancer cell PC3, LNcaP, DU145, human intestinal cancer cell SW480, HT-2P . The MTT method (reference Mosmann T. Rapid colorimetric assay for cellular growth and survival-application to proliferation and cytotoxicity assays. J Immunol Methods, 1983, 65(1-2), 55-63.) was used to determine the concentration of each tumor cell. The half-inhibitory concentration IC when the survival rate (or mortality rate) is 50% 50 . As normal cells, human kidney epidermal cells HKC-8 w...
Embodiment 2
[0040] Selective tumor suppressor effect of bixanthone compound FLBG-1108
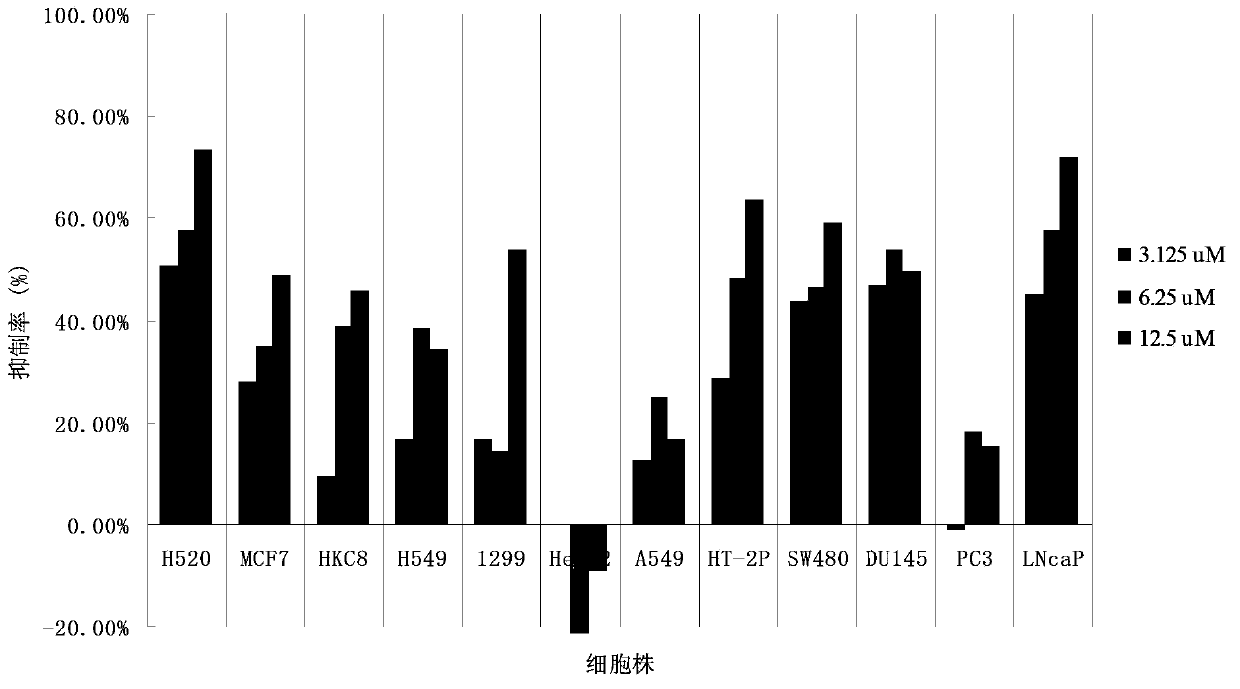
[0041] The test method (MTT) is similar to Example 1, using the same human cancer cell line and normal renal epidermal cells, the administration concentration of FLBG-1108 is set at three concentration gradients of 3.125, 6.25, and 12.5 μM, and mixed with various cells After 48 h of culture, the viability of the cells was measured.
[0042] Further, three concentration gradients (3.125, 6.25, 12.5μM) were used to measure the selective inhibitory effect of FLBG-1108 on various cancer cells. The results are as follows: figure 1 shown. The diketone (bixanthone) compound FLBG-1108 showed inhibitory effects on the proliferation of human lung cancer cells H520, H549, 1299, and A549, but it showed obvious selective inhibitory effects on human lung cancer cells H520. The inhibitory rate was gradually enhanced with the increase of the concentration, and the inhibitory degree was obviously stronger than that o...
Embodiment 3
[0044] Dose-effect relationship of bixanthone compound FLBG-1108 in inhibiting the growth of lung cancer cell line H520
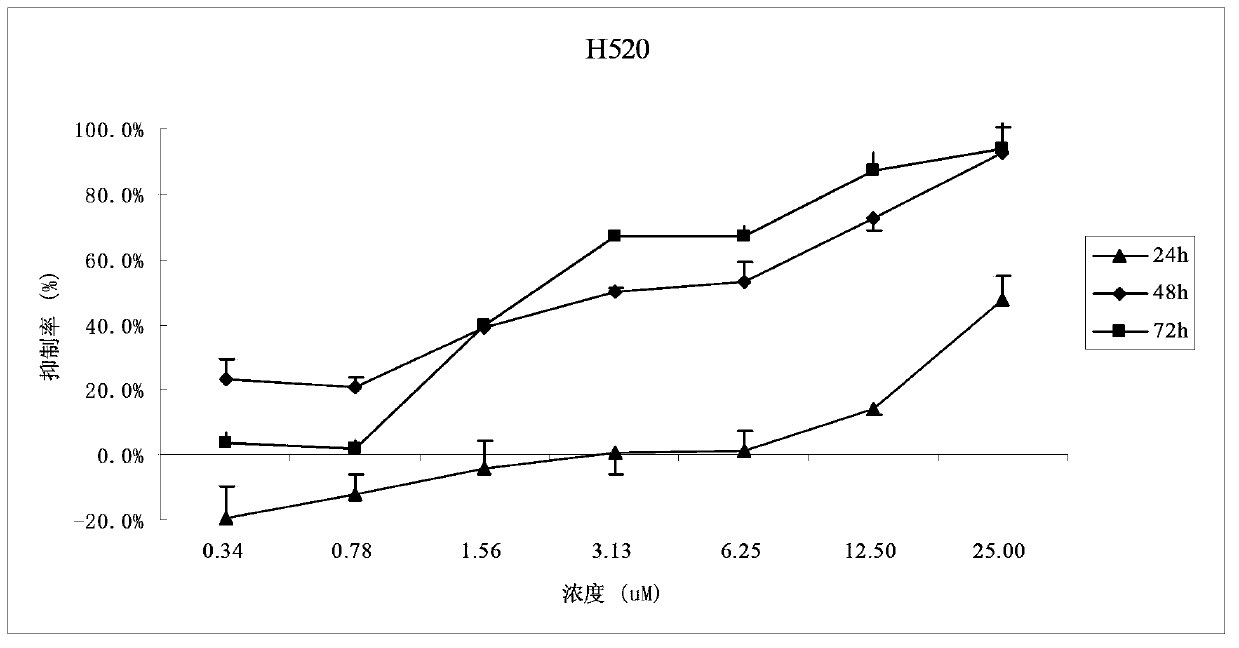
[0045] The method is similar to that of Example 1, and the human lung cancer cell line H520 is mainly used for determination, and the administration concentration is set at seven concentration gradients of 0.34, 0.78, 1.56, 3.12, 6.25, 12.5, and 25.0 μM, and the drug is mixed with the cancer cell H520 for 24 hours. After 48h and 72h, the survival rate of cancer cells was measured respectively.
[0046] The dose-effect relationship of FLBG-1108 inhibiting the growth of lung cancer cell H520 is as follows: figure 2 As shown, the results show that when the bixanthone compound FLBG-1108 acts on lung cancer cell H520 for 24 hours, its inhibitory activity is weak, but its inhibitory activity is significantly enhanced after 48 hours and 72 hours, showing a good concentration and time dependent effect. Especially after 48h and 72h of action, there is a good line...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com