Calcium-sensing receptor-active compounds
A compound and stereoisomer technology, applied in the field of calcium-sensing receptor activating compounds, can solve problems such as women's influence, achieve the effects of prolonging the half-life in vivo, prolonging the duration of curative effect in vivo, and increasing the distribution volume in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
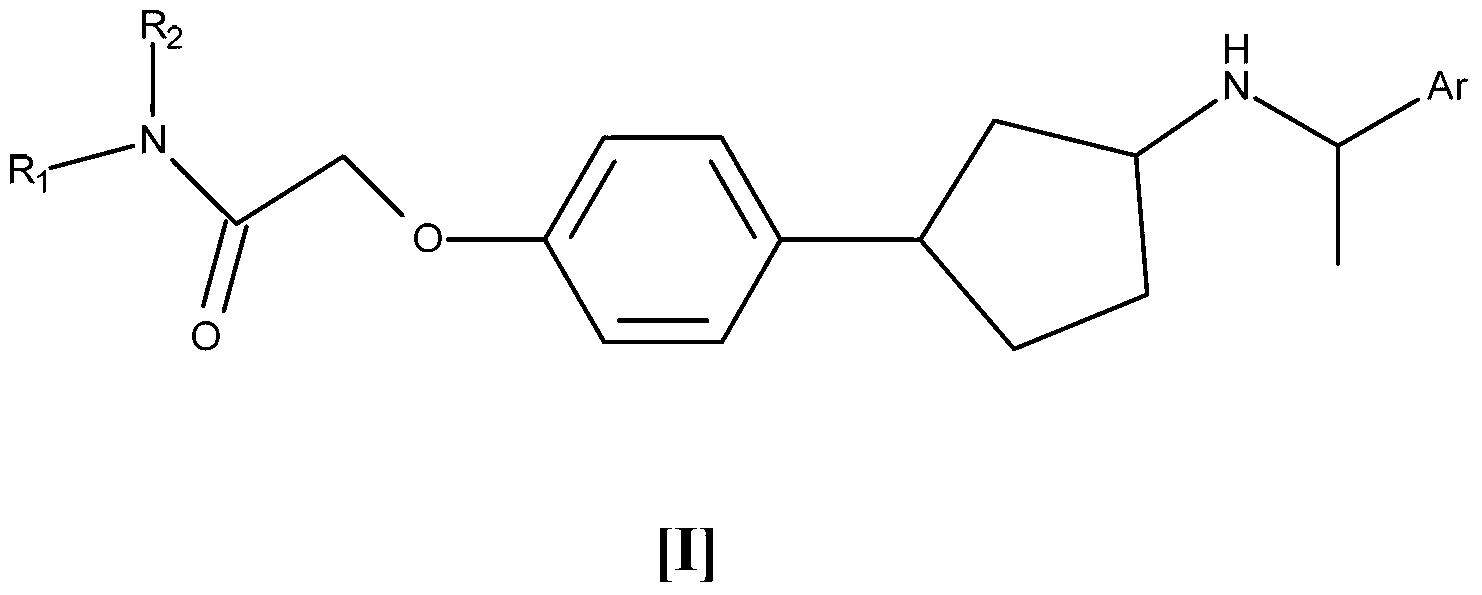
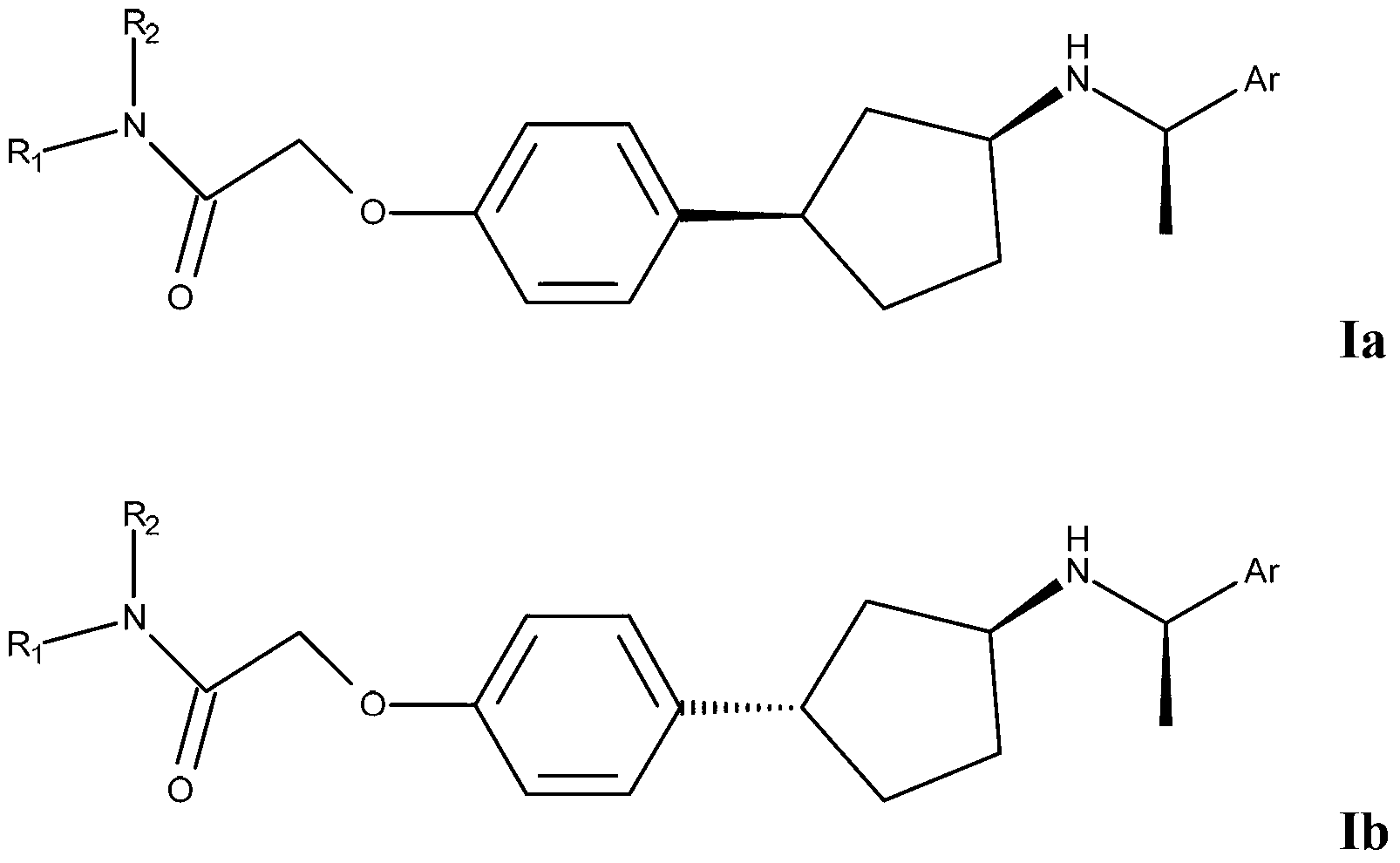
[0056] In an embodiment of the invention, compound I is represented as Ia or Ib
[0057]
[0058] In one embodiment of the present invention, Ar represents phenyl or naphthyl, optionally replaced by one or two, the same or different, selected from halogen or C 1-3 Alkoxy substituents are substituted.
[0059] In one embodiment of the invention, Ar represents phenyl, which is substituted by 1 or 2, the same or different, substituents selected from chlorine, fluorine or methoxy.
[0060] In one embodiment of the invention Ar represents 4-fluoro-3-methoxy or 3-chlorophenyl.
[0061] In one embodiment of the invention Ar represents naphthyl.
[0062] In one embodiment of the invention, R 1 stands for C 2-4 Alkenyl, hydroxyl C 2-4 Alkyl, hydroxyl C 2-4 Alkylamino C 2-4 Alkyl, C 1-3 Alkylsulfonylamino C 2-4 Alkyl, aminosulfonyl C 1-4 Alkyl, aminocarbonyl C 1-4 Alkyl or C containing 1-2 heteroatoms selected from N, O and S 2-5 Heterocycloalkyl.
[0063] In one embodim...
Embodiment 1
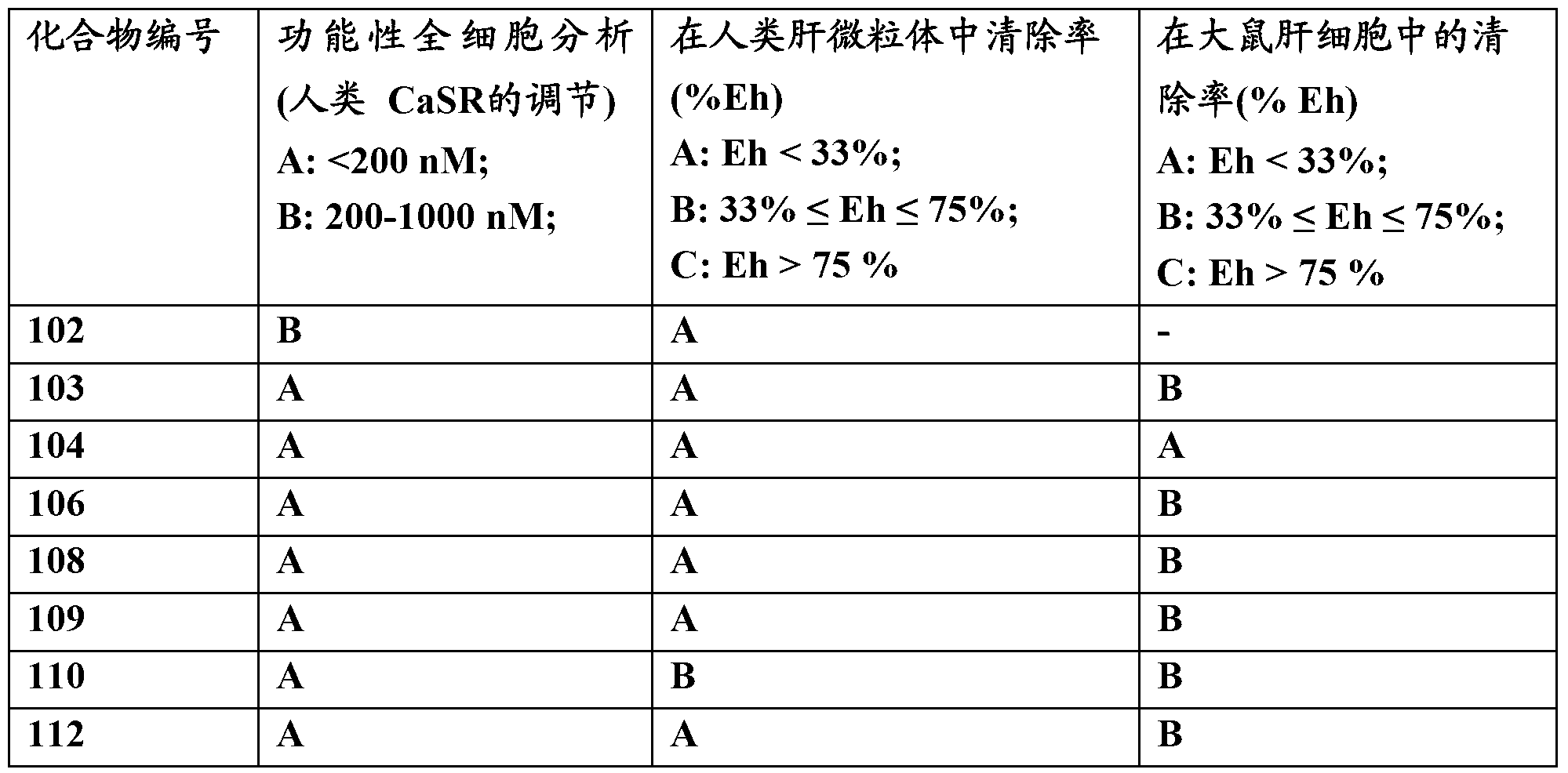
[0255] Example 1: 4-[2-[4-[(1R,3S)-3-[[(1R)-1-(4-fluoro-3-methoxy-phenyl)ethyl]amino]cyclopenta Base] phenoxy] acetyl] piperazin-2-one (compound 101)
[0256] Following general procedure A, intermediate 1 was employed as the acid and piperazin-2-one was employed as the amine.
[0257] 1 H NMR (600MHz, DMSO) δ8.14 / 8.09 (s, 1H, rotamer), 7.16 (dd, J=8.6, 1.8Hz, 1H), 7.14–7.07 (m, 3H), 6.88 (ddd, J=8.1,4.3,1.9Hz,1H), 6.82(d,J=8.2Hz,2H), 4.80 / 4.78(s,2H, rotamer), 4.07 / 3.94(s,2H rotamer) , 3.82(s,3H), 3.75(q,J=6.5Hz,1H), 3.66–3.62 / 3.62–3.57(m,2H, rotamer), 3.29–3.25 / 3.20–3.15(m,2H, rotamers), 2.91–2.85(m,1H), 2.85–2.77(m,1H), 2.05–1.98(m,1H), 1.89–1.83(m,1H), 1.82–1.74(m,1H) , 1.65–1.52(m,2H), 1.32–1.24(m,1H), 1.23(d,J=6.6Hz,3H).
Embodiment 2
[0258] Example 2: 2-[4-[(1R,3S)-3-[[(1R)-1-(3-chlorophenyl)ethyl]amino]cyclopentyl]-phenoxy]-N -(4-piperidinyl)-acetamide (compound 102)
[0259] Following general procedure B, intermediate 6 was employed as the ketone and (R)-1-(3-chlorophenyl)ethanamine was employed as the amine. The BOC-protected intermediate was treated with HCl in methanol for 2 h, followed by evaporation of the solvent to obtain the target compound.
[0260] 1H NMR (600MHz, DMSO) δ7.97–7.87(m,1H, rotamer), 7.43–7.41(m,1H), 7.35–7.29(m,2H), 7.27–7.23(m,1H), 7.17–7.08(m,2H), 6.86–6.81(m,2H), 4.41–4.37(m,2H), 3.77(q,J=6.6Hz,1H), 3.71–3.63(m,1H), 2.94– 2.88(m,2H), 2.88–2.78(m,2H), 2.49–2.43(m,2H), 2.04–1.98(m,1H), 1.89–1.82(m,1H), 1.79–1.72(m,1H ), 1.67–1.53(m,4H), 1.37–1.24(m,3H), 1.23(d,J=6.6Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com