An anti-tumor targeting complex and a preparation method and applications thereof
A targeting complex, anti-tumor technology, applied in anti-tumor drugs, chemical instruments and methods, hybrid peptides, etc., can solve problems such as insufficient killing of tumor cells and failure to achieve anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Recombinant expression of mature CTX in Escherichia coli
[0024] Induced expression and isolation and purification of GST-6×His-CTX: The gene encoding the CTX precursor carrying 6×His tag and enterokinase cleavage site at the N-terminus was synthesized by chemical method, using E. coli preferred codons (Seq ID NO .1). The chemically synthesized gene was cloned into the Escherichia coli expression vector pGEX-4T-1, and confirmed by DNA sequencing. The expression vector pGEX-4T-1 / 6×His-CTX was transformed into Escherichia coli BL21(DE3), and its expression was induced by IPTG in TB medium (cultured overnight at 16°C). The bacteria were collected by centrifugation and resuspended in lysis buffer (50mmol / l phosphate, 0.5mol / l NaCl, pH 7.5). The bacteria were broken by pressure method, and DNase I was added to degrade DNA. Then add solid sodium sulfite to a final concentration of 0.2 mol / L, add solid sodium tetrathionate to a final concentration of 0.15 mol / L, ...
Embodiment 2
[0026] Example 2: Recombinant expression of mature Onc in Escherichia coli
[0027] 6×His-Onc induced expression and isolation and purification: The Onc gene carrying 6×His tag at the N-terminus was synthesized by chemical method, and the preferred codon of E. coli was selected (Seq ID NO.3). The gene was ligated into the expression vector pET and confirmed by DNA sequencing. Then the expression vector pET / 6×His-Onc was transformed into Escherichia coli BL21(DE3), and the expression was induced with IPTG in TB medium at 37°C. The bacteria were disrupted by ultrasonic method, and the inclusion bodies were collected by centrifugation. The inclusion bodies were dissolved with 6 mol / l guanidine hydrochloride, and the final concentration of 0.2 mol / l sodium sulfite and 0.15 mol / l sodium tetrathionate was added to reversibly modify the sulfhydryl group in Onc. After shaking slowly at room temperature for 2-3h, the sulfonated 6×His-Onc was purified by metal chelate chromatography, ...
Embodiment 3
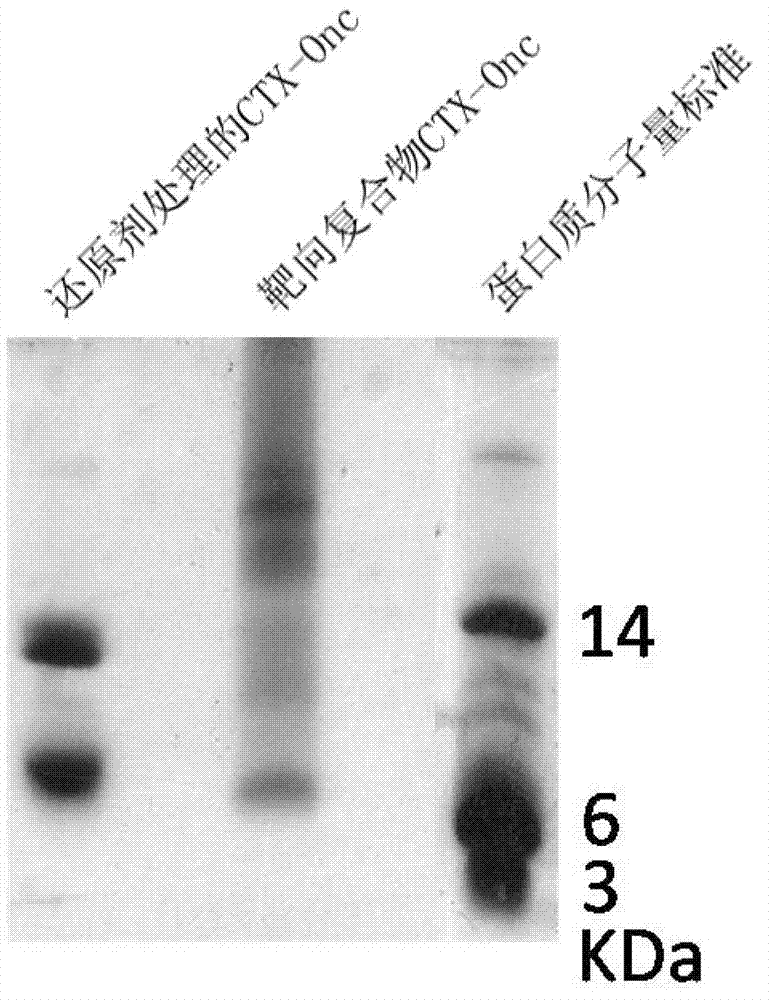
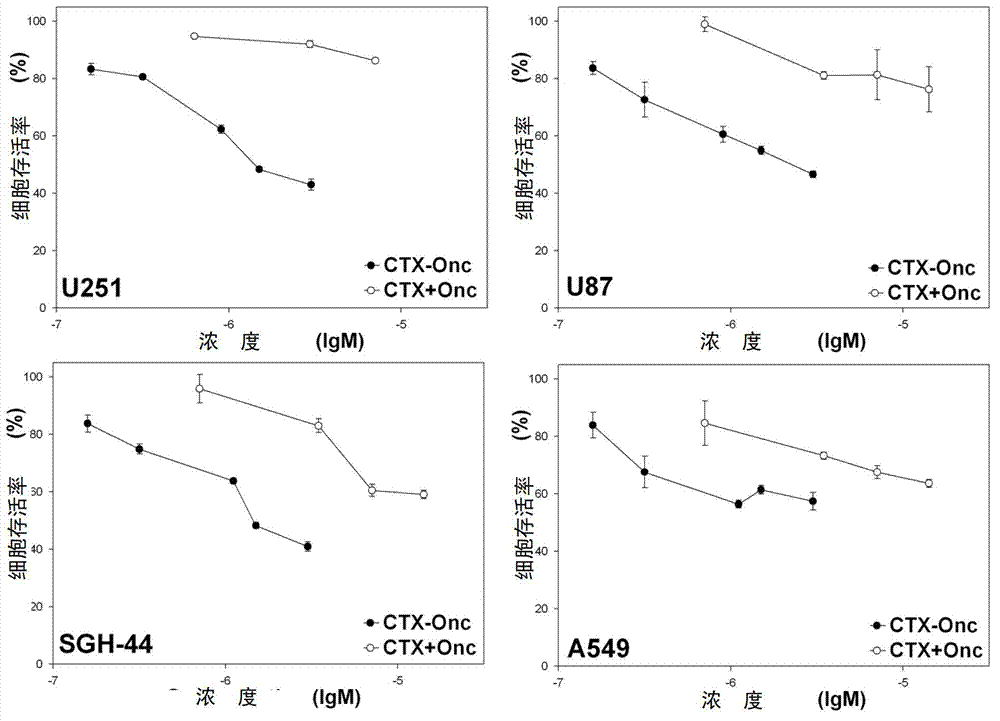
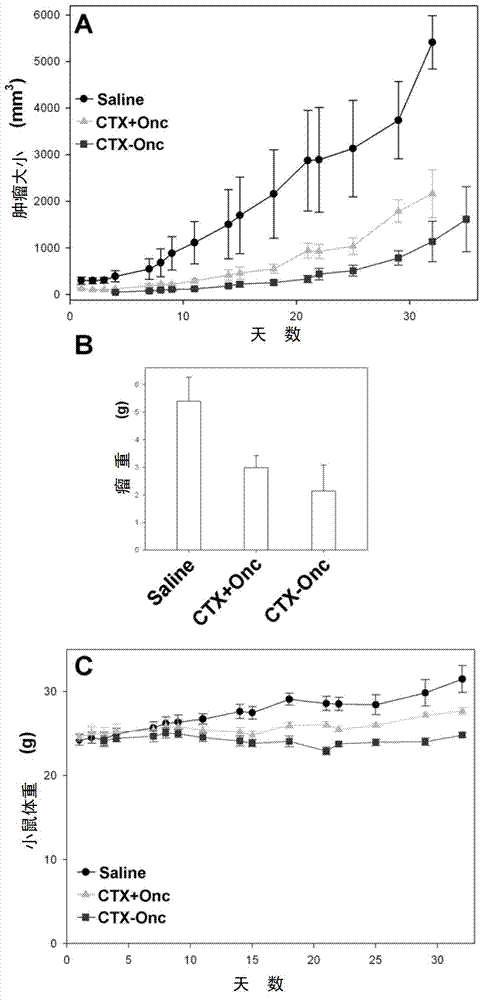
[0029] Example 3: Chemical crosslinking of CTX and Onc
[0030]The mature CTX recombinantly prepared in Example 1 and the amino-specific modifier SPDP were reacted in phosphate buffer (50mmol / l sodium phosphate, 150mmol / l NaCl, pH8.0) at room temperature for 1 hour at a molar ratio of 1:1, and then Adjusted to pH 3.0 with TFA, purified by HPLC, and the modified CTX was collected and identified by mass spectrometry. The mature Onc prepared recombinantly in Example 2 and the amino-specific modifier 2-Iminothiolane were mixed in phosphate buffer (50mmol / l sodium phosphate, 150mmol / l NaCl, 2.4mmol / l mercaptoethanol, pH8.0) at a ratio of 1:20 The molar ratio was reacted at room temperature for 1 h, then the pH was adjusted to 3.0 with acetic acid, and the excess modifier was removed with Sephadex G-25 molecular sieve. The above-mentioned modified CTX and modified Onc were reacted at room temperature for 0.5 h in phosphate buffer (50 mmol / l sodium phosphate, 150 mmol / l NaCl, pH 8.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com