Application of disulfiram in preparing anti-liver cirrhosis or anti-liver fibrosis pharmaceuticals
An anti-fibrosis and liver fibrosis technology, applied in drug combination, microbiological determination/testing, digestive system, etc., can solve problems such as not revealing the anti-cirrhosis and anti-fibrosis effects of disulfiram
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Establishment of rat liver fibrosis model
[0038] 1) Test material:
[0039] Disulfiram (DSF): purchased from Sigma-Aldrich company;
[0040] Carbon tetrachloride (CCL4) and olive oil: purchased from Sinopharm Chemical Reagent Co., Ltd.
[0041] 2) Control group: get 6 3-4 week male Sprague-Dawley (SD) rats, intraperitoneally inject 50% carbon tetrachloride (prepared with olive oil), injection amount 2ml / kg body weight, 2 times a week, a total of 8 Zhou, established rat liver fibrosis animal model.
[0042] 3) Administration group: get 5 male Sprague-Dawley (SD) rats of 3-4 weeks, CCl 4 Disulfiram was administered at the same time, and its amount was injected intraperitoneally at 8 mg / kg body weight, twice a week.
Embodiment 2
[0043] Example 2: Preparation of Serum and Liver Specimens
[0044] 1) Serum preparation: After 8 weeks, the rats were sacrificed. The blood was placed in a 4°C refrigerator overnight, and the next day, the supernatant was drawn and centrifuged at 3000 rpm for 20 minutes, the supernatant was carefully drawn, and frozen at -20°C.
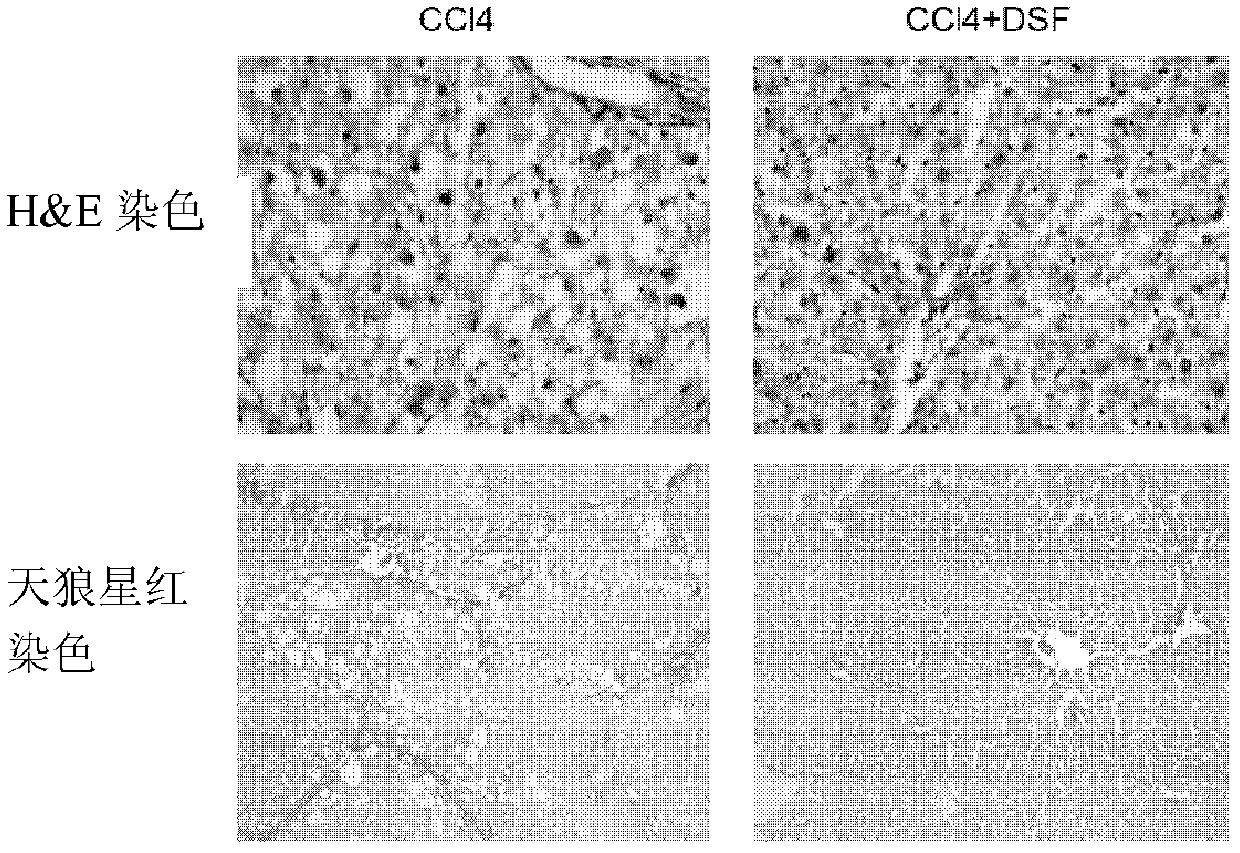
[0045] 2) Liver treatment: the left liver lobe was cut and placed in a cryopreservation tube, immediately placed in liquid nitrogen, and then placed at -80°C for RNA extraction and cytokine determination. After the remaining liver was washed twice in PBS, it was put into a 50ml centrifuge tube and fixed with paraformaldehyde solution for paraffin sectioning.
Embodiment 3
[0046] Embodiment 3: detection of serum ALT and AST
[0047] Alanine aminotransferase (ALT) kit and aspartate aminotransferase (AST) kit were purchased from Shanghai Shensuo Youfu Medical Diagnostic Products Co., Ltd.
[0048] a) Take a 96-well plate, add 7.5 μl of rat serum respectively, and make three replicate wells for each sample. Then add 150 μl of the R-1 solution in the kit to the sample well, mix well and react for 5 minutes;
[0049] b) Add 50 μl of the R-2 solution in the kit and mix;
[0050] c) Measure the absorbance A1 at 340nm wavelength after 1 minute;
[0051] d) Measure the absorbance A2 at 340nm wavelength again after 4 minutes;
[0052] e) Calculation of rat serum ALT value:
[0053] ALT(U / L)=(A1-A2) / 4 minutes×207.5μl×1000 / (6.22×7.5μl×1cm)
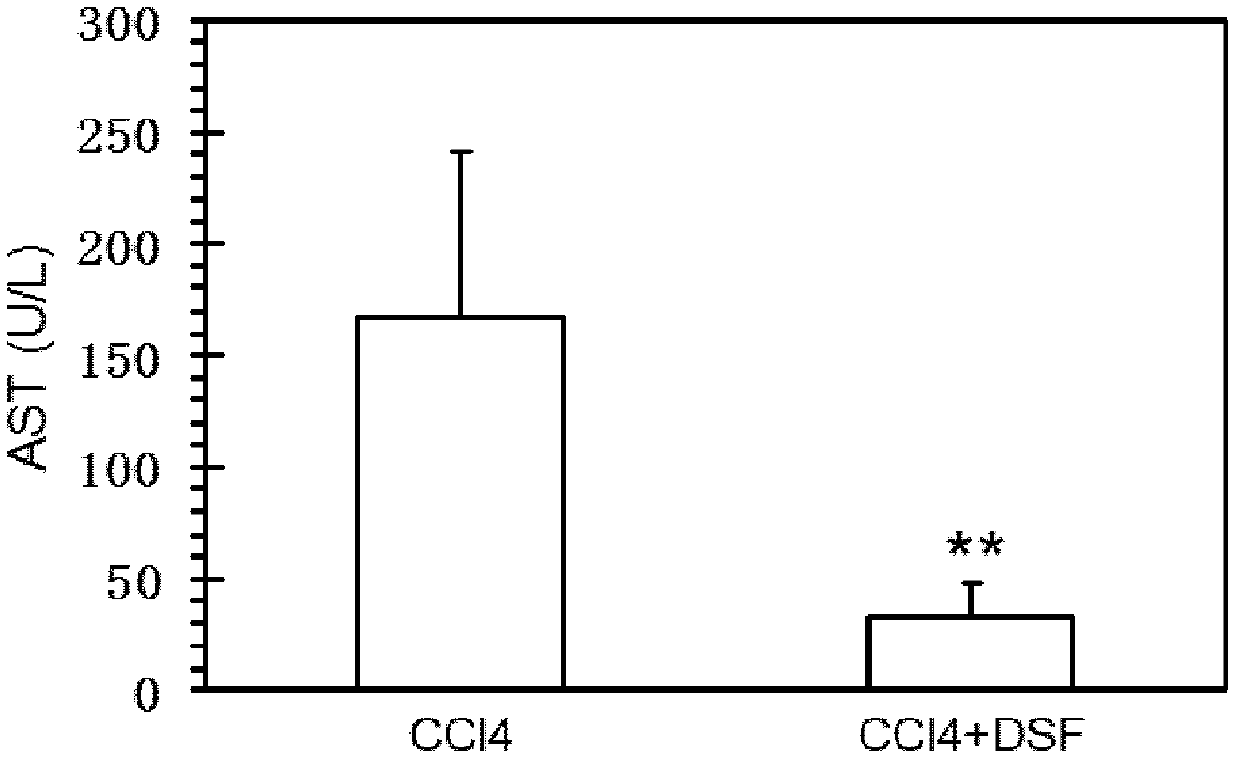
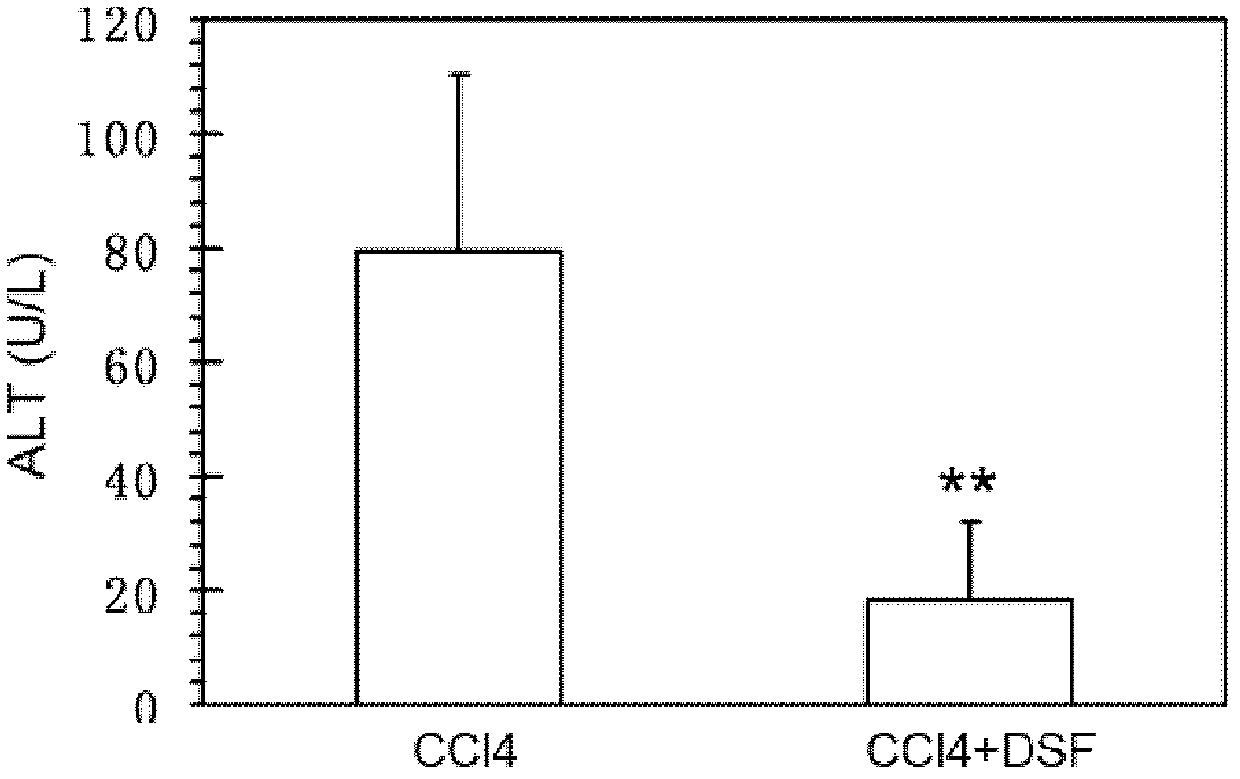
[0054] see results Figure 1A with Figure 1B , these figures show that CCl 4 +DSF treatment group (i.e. administration group) serum ALT and AST levels will be significantly lower than CCl 4 The treatment group ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com