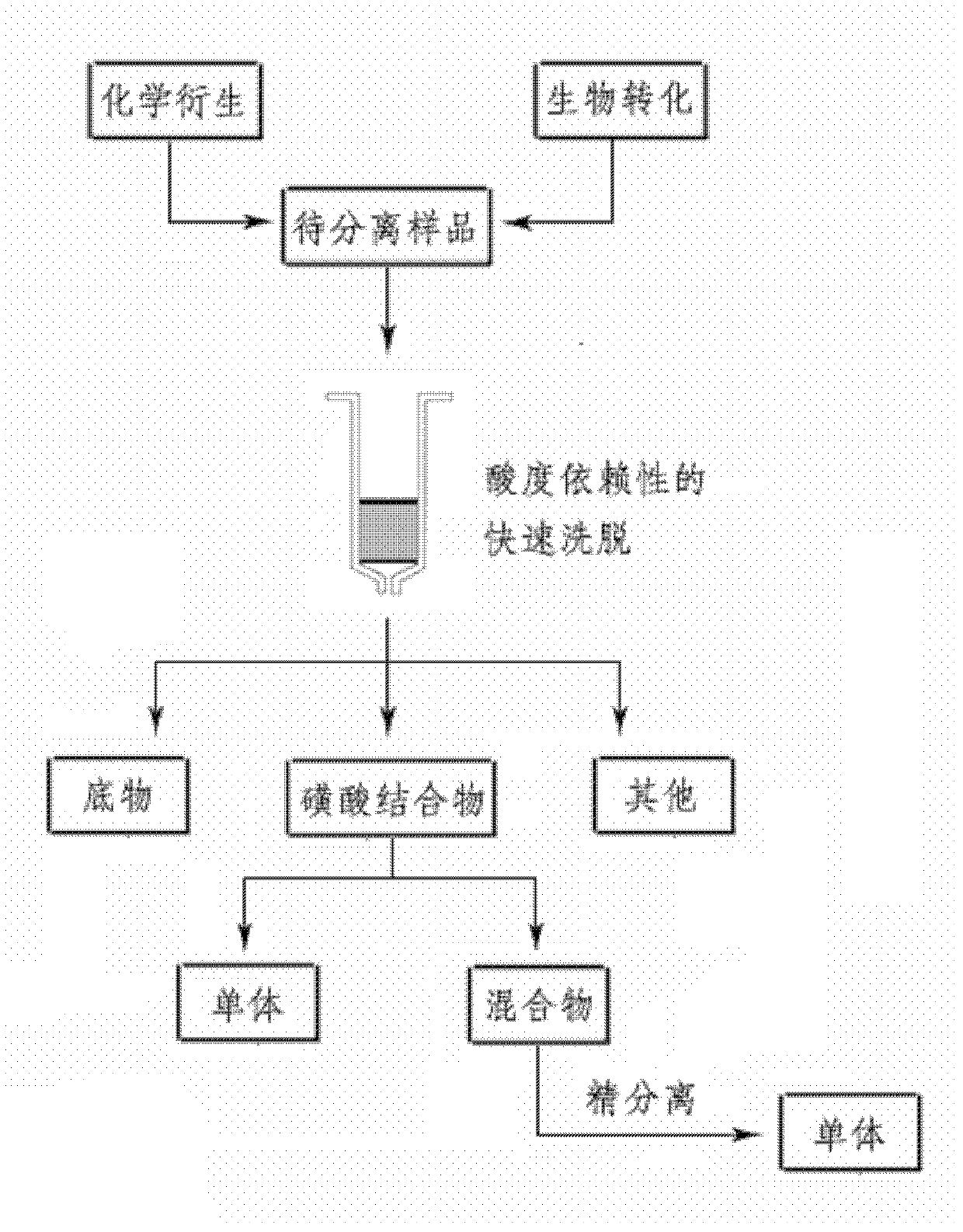
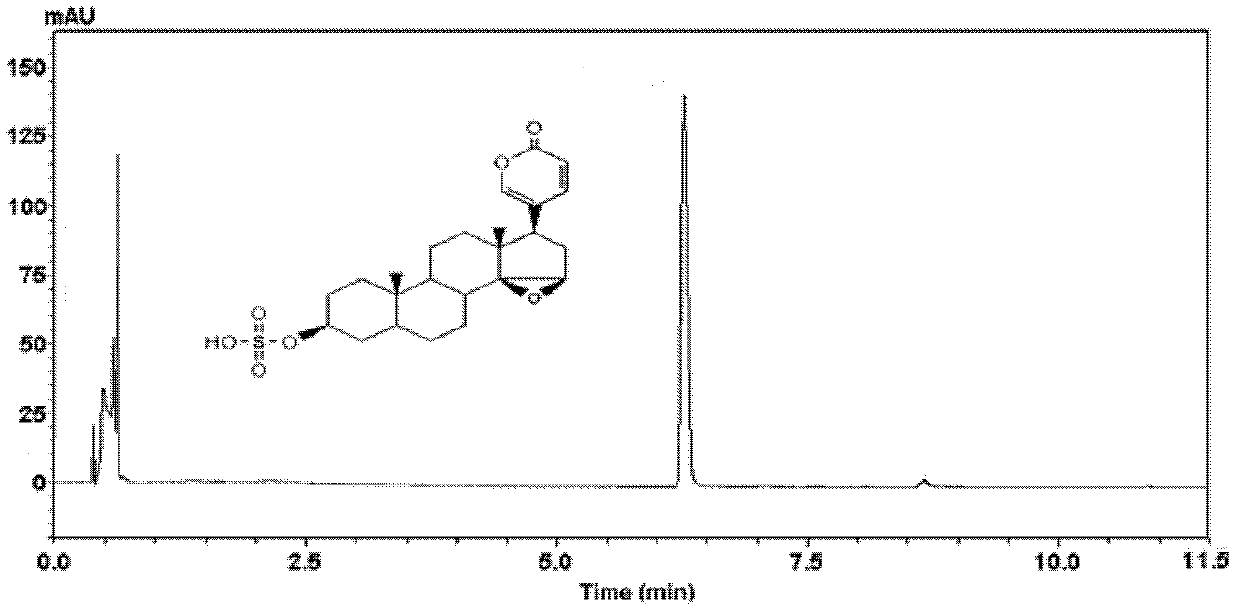
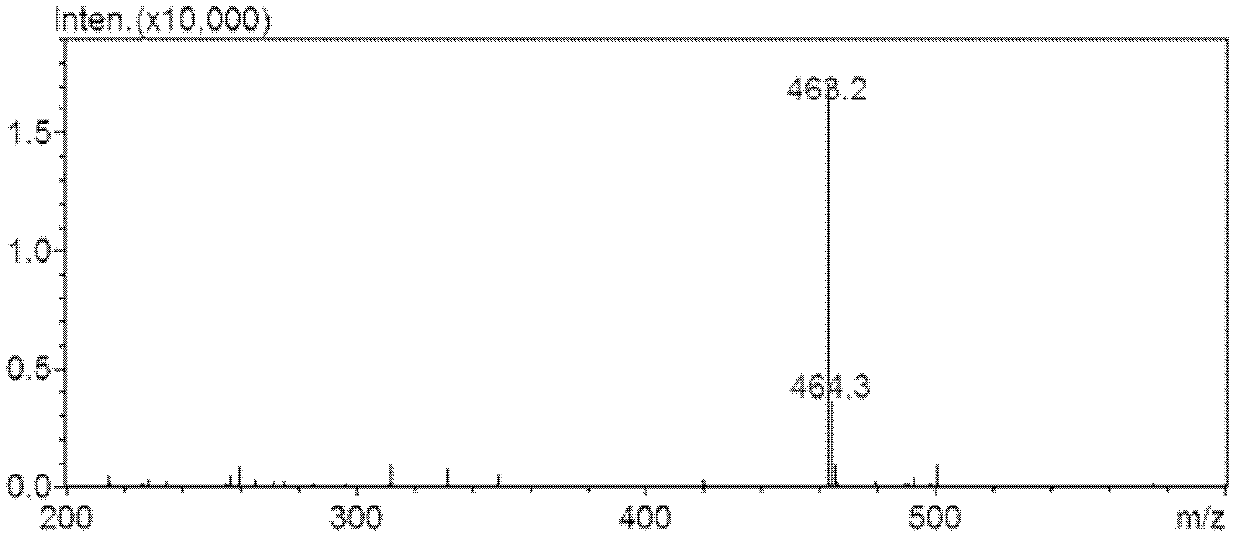
Separation method of sulfonic acid combination
A technology of sulfonic acid conjugate and separation method, applied in the field of preparation of sulfonic acid conjugate, can solve the problem of lack of an effective method for rapid separation of sulfonic acid conjugate monomer, and achieve wide applicability, simple and efficient operation, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the preparation of pregnenol ketone sulfonic acid conjugate, as follows:
[0024]
[0025] 1) Preparation of isolated samples by biotransformation method: Pregnenolone was dissolved in methanol to form a 5mg / ml stock solution, 20ml was taken out and phosphate buffer salt of pH 7.4 was added, after stirring, rat liver cytoplasm ( RLC), adding sulfonic acid group donor PAPS, the final concentration of bufagenin in the system is 0.10mg / ml, the final concentration of protein is 0.5mg / ml, the final concentration of PAPS is 0.2mM, and incubated in a constant temperature water bath at 37°C for 2h. After the substrate conversion rate reached 50% as detected by HPLC, the reaction solution was transferred to a -80°C refrigerator for cooling. Take 100ml of the reaction solution and put it directly on the flash chromatography column. The filler of the flash chromatographic column is a mixed filler, wherein the silica gel is bonded with phenyl groups, the particle ...
Embodiment 2
[0027] Embodiment 2, the preparation of esterbufaxin sulfonic acid conjugates, as follows:
[0028]
[0029] Bufagenin was dissolved in dimethyl sulfoxide (DMSO) to make a 5 mg / ml stock solution, 20 ml was taken out and phosphate buffer salt of pH 7.4 was added, after stirring, human recombinant monoenzyme rhSULT2A1 was added in turn, and sulfonium The acid group donor PAPS, the final concentration of bufagenin in the system is 0.10mg / ml, the final concentration of protein is 0.25mg / ml, the final concentration of PAPS is 0.2mM, and the mixture is stirred and incubated in a constant temperature water bath at 37°C for 2h. After the substrate conversion rate reached 50% as detected by HPLC, the reaction solution was transferred to a -80°C refrigerator for cooling. Take 100ml of the reaction solution and put it directly on the flash chromatography column. The filler of the flash chromatographic column is a mixed filler, wherein the silica gel is bonded with phenyl groups, the ...
Embodiment 3
[0030] Example 3, Preparation of daphnetin sulfonated metabolites
[0031]
[0032] Daphnetin was dissolved in methanol to make a 5mg / ml stock solution, 30ml was taken out and added to phosphate buffer salt with pH 7.4, after stirring, it was added to rat intestine S9 (RIS9) in turn, and PAPS, a sulfonic acid group donor, was added to the system The final concentration of daphnetin was 0.10 mg / ml, the final concentration of protein was 0.5 mg / ml, the final concentration of PAPS was 0.2 mM, and the mixture was stirred and incubated in a constant temperature water bath at 37°C for 1 hour. After the substrate conversion rate reached 50% as detected by HPLC, the reaction solution was transferred to a -80°C refrigerator for cooling. Take 100ml of the reaction solution and put it directly on the flash chromatography column. The filler of the flash chromatographic column is a mixed filler, in which silica gel is bonded with phenyl groups, the particle size is 40 μm, and the dosage ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com