Chitosan oligosaccharide kojic acid derivative and preparation method thereof
A technology of chitooligosaccharide kojic acid and derivatives, applied in the field of food additives, can solve the problems of complex derivatization process of chitooligosaccharide, failure to meet application requirements, unfavorable large-scale production, etc., to achieve environmental protection, low toxicity, The effect of broad application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
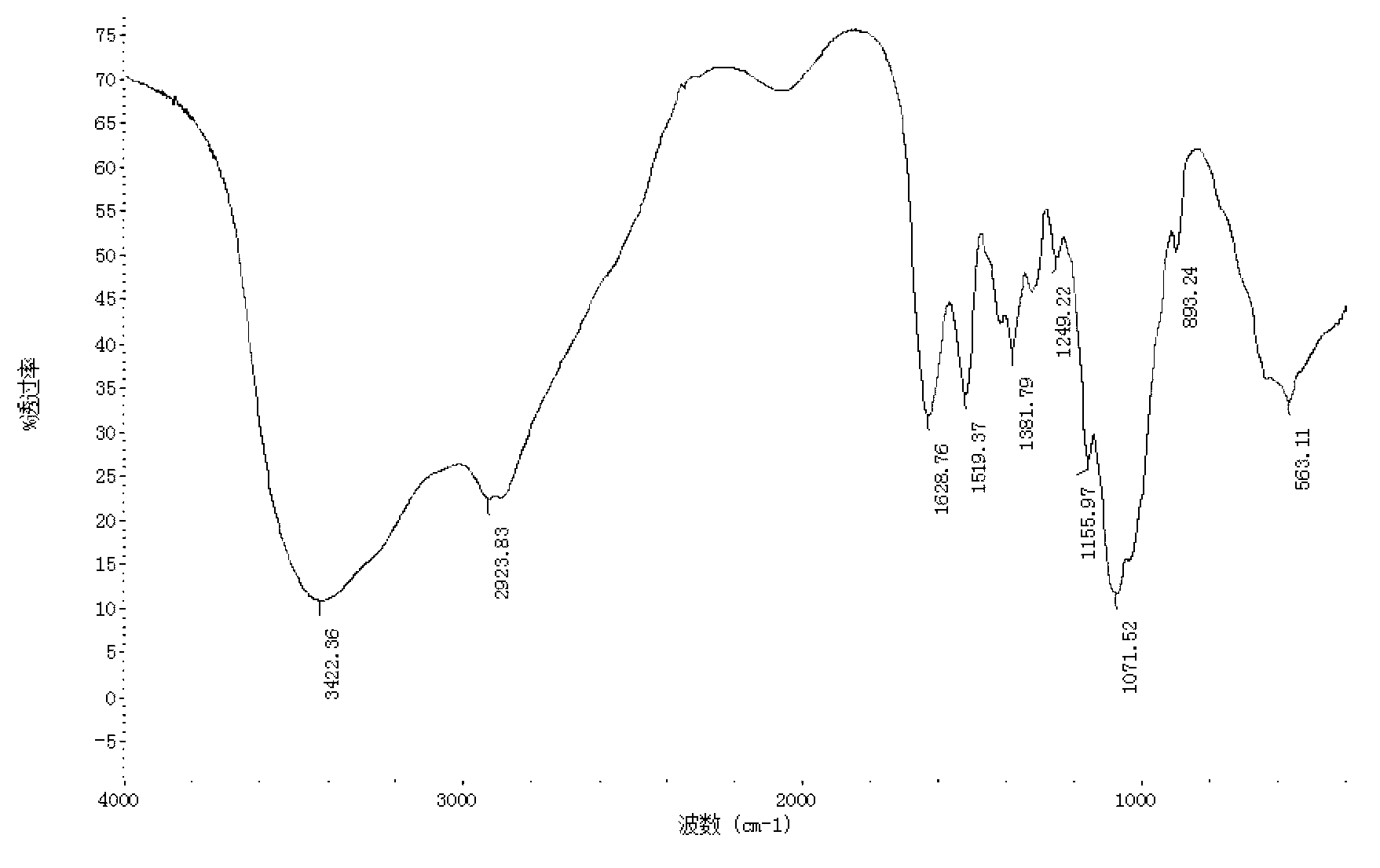
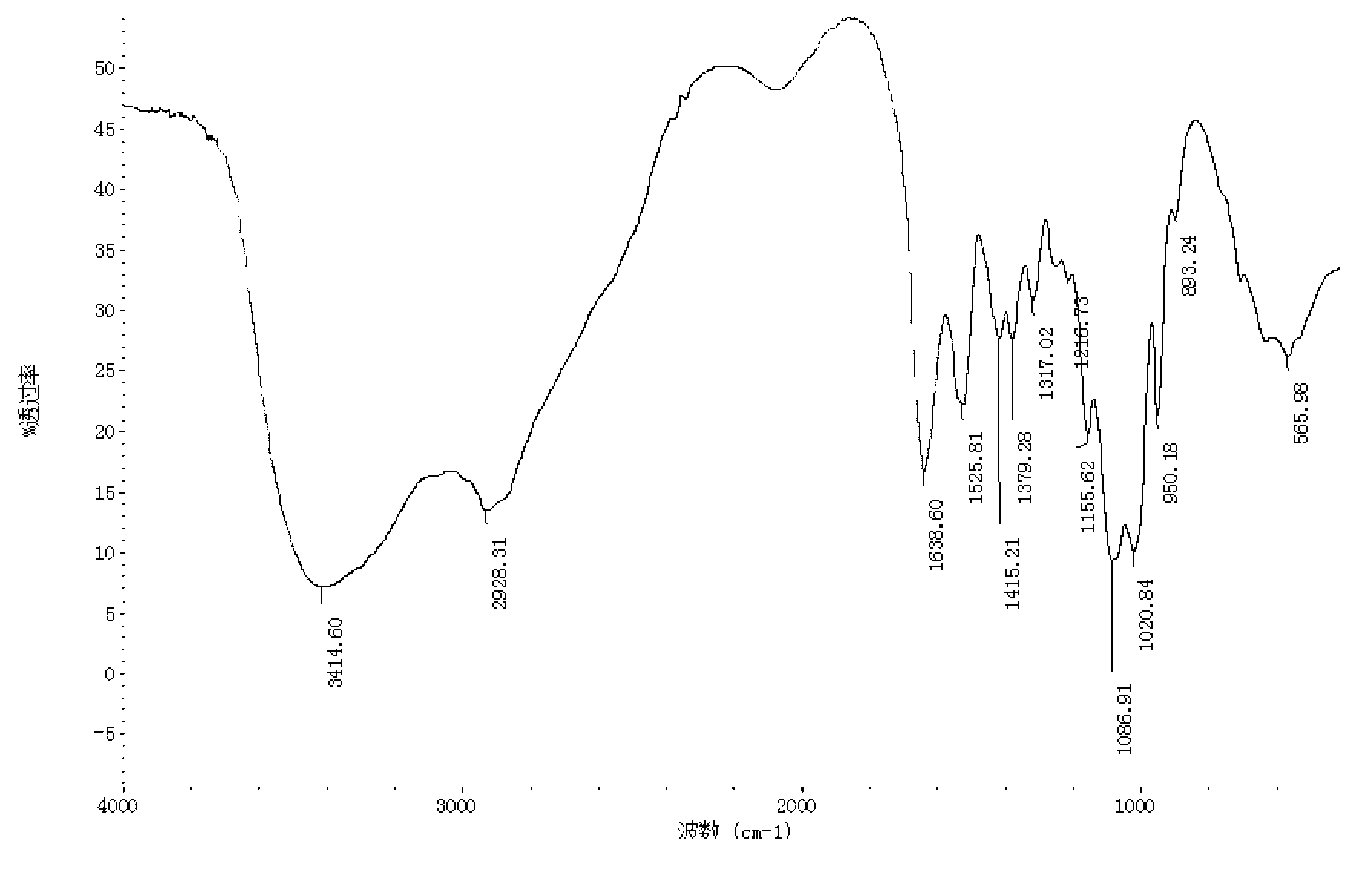
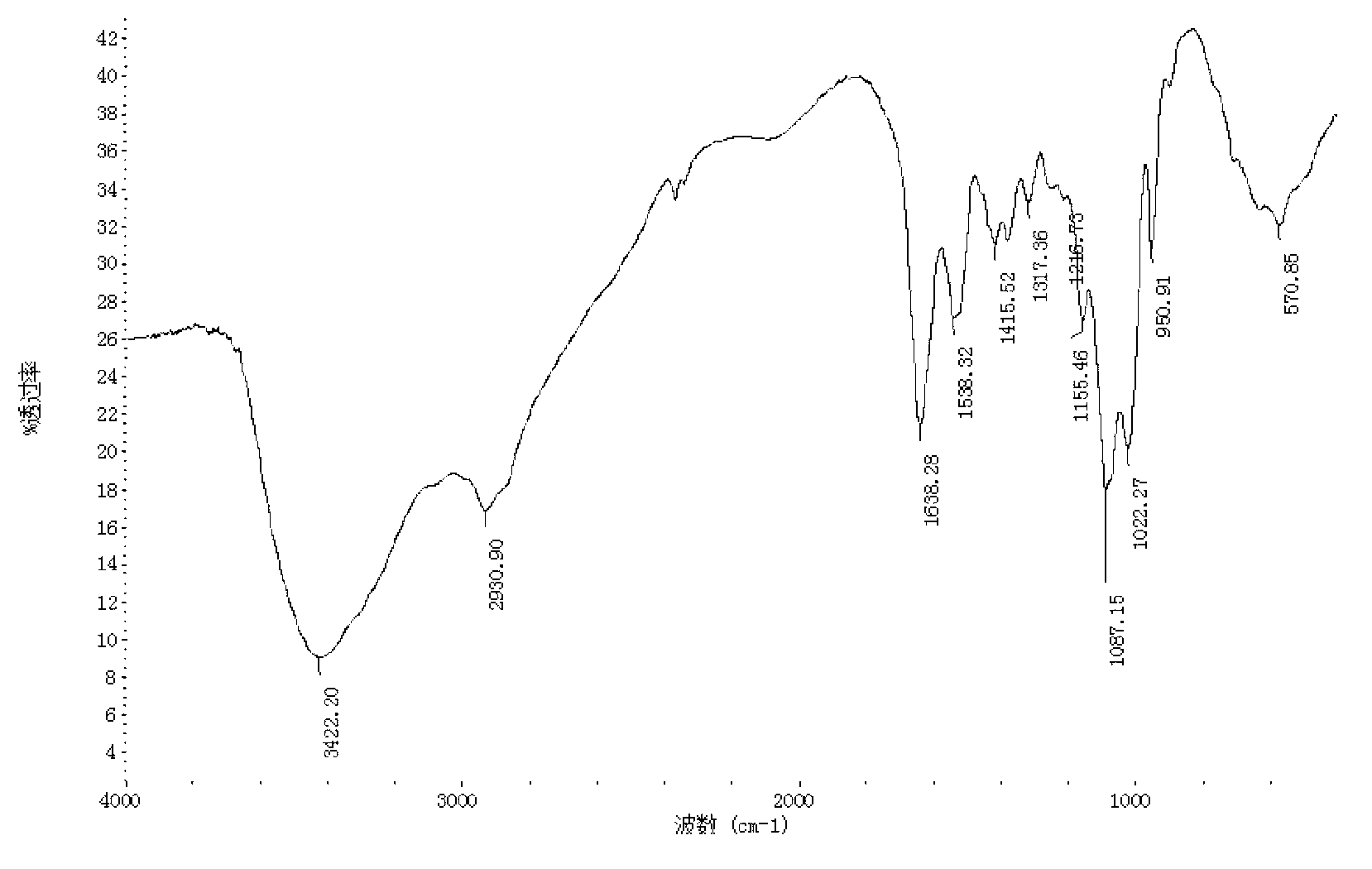
[0030] Under stirring, dissolve 1 g of kojic acid in 2 mL of dimethyl sulfoxide, and dissolve 0.5 g of chitosan oligosaccharide (molecular weight is 1000 Da, deacetylation degree is 90%) in 2 mL of dimethyl sulfoxide and 2 mL of pyridine mixture. In , the chitosan oligosaccharide dissolved in the mixture of dimethyl sulfoxide and pyridine was added dropwise to the kojic acid dissolved in dimethyl sulfoxide, and the reaction mixture was magnetically stirred in a water bath at 25°C for 4 hours, then poured into excess Acetone precipitates the product, the precipitate is filtered, the precipitate is subjected to Soxhlet extraction with ethanol and acetone respectively to remove dimethyl sulfoxide and pyridine, and finally the product is vacuum freeze-dried. identified by infrared spectroscopy 1 H NMR identified its structure. The yield of the final product was 46%.
Embodiment 2
[0032]Under stirring, dissolve 1 g of kojic acid in 3 mL of dimethyl sulfoxide, and dissolve 0.5 g of chitosan oligosaccharide (molecular weight of 3000 Da, deacetylation degree of 95%) in 3 mL of dimethyl sulfoxide and 4 mL of pyridine mixture. In the method, the chitosan oligosaccharide dissolved in the mixture of dimethyl sulfoxide and pyridine was added dropwise to the kojic acid dissolved in dimethyl sulfoxide, and the reaction mixture was magnetically stirred in a water bath at 35°C for 6 hours, then poured into excess Acetone precipitates the product, the precipitate is filtered, the precipitate is subjected to Soxhlet extraction with ethanol and acetone respectively to remove dimethyl sulfoxide and pyridine, and finally the product is vacuum freeze-dried. identified by infrared spectroscopy 1 H NMR identified its structure. The yield of the final product was 52%.
Embodiment 3
[0034] Under stirring, dissolve 0.5 g of kojic acid in 4 mL of dimethyl sulfoxide, 0.5 g of chitosan oligosaccharide (molecular weight of 5000 Da, deacetylation degree of 90%) in 4 mL of dimethyl sulfoxide and 6 mL of pyridine and mix In the solution, the chitosan oligosaccharide dissolved in the mixture of dimethyl sulfoxide and pyridine was added dropwise to the kojic acid dissolved in dimethyl sulfoxide, and the reaction mixture was magnetically stirred in a water bath at 45°C for 8 hours, then poured into Excessive acetone causes the product to precipitate, the precipitate is filtered, the precipitate is subjected to Soxhlet extraction with ethanol and acetone respectively to remove dimethyl sulfoxide and pyridine, and finally the product is vacuum freeze-dried. identified by infrared spectroscopy 1 H NMR identified its structure. The yield of the final product was 58%.
[0035] Comprehensive embodiment 1~3, the present invention dissolves chitosan oligosaccharide and ko...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com