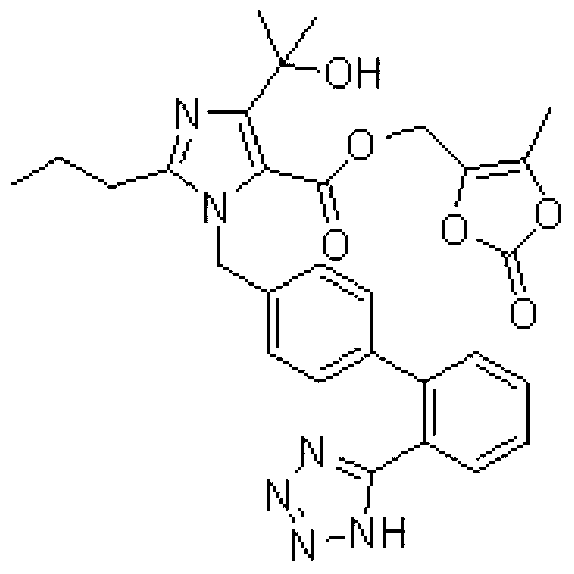
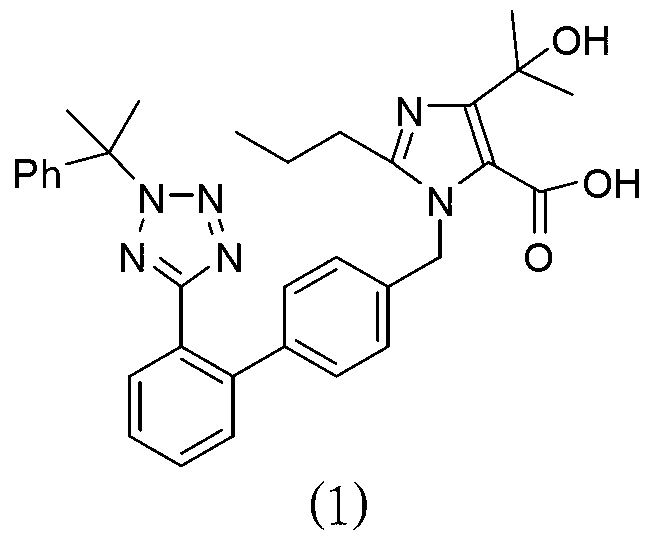
Preparation method of olmesartan medoxomil intermediate and synthesis method of olmesartan medoxomil
A technology of olmesartan medoxomil and synthesis method, which is applied in the field of olmesartan medoxomil synthesis, can solve problems such as high price, and achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation of formula (2) compound
[0054] Step 1: Preparation of the compound of formula (2-1)
[0055] Chemical name of compound of formula (2-1): 5-(4'-methylbiphenyl-2-yl)tetrazole.
[0056] The structural formula of the compound of formula (2-1) is as follows:
[0057]
[0058] Its preparation process: 19.3g (about 0.1mol) of 2-cyano-4'-methylbiphenyl (that is, the compound of formula (2-0)), 9.8g (about 0.15mol) of sodium azide dissolved in 100g of dimethyl Add 4.5 g of sodium bisulfate silica gel to base formamide, and react at 120° C. for 10 hours. The reaction mixture was cooled to room temperature, filtered, and the filter cake was washed with 100 mL×2 ethyl acetate. Add 600mL ethyl acetate and 400mL 4mol / L hydrochloric acid to the filtrate, and stir vigorously. The organic layer was separated, and the aqueous layer was extracted with 200 mL×2 ethyl acetate. The organic layers were combined, washed with 200 mL×2 water, and concentra...
Embodiment 2
[0083] Embodiment 2: the preparation of formula (3) compound
[0084] Step 1: Preparation of the compound of formula (3-1)
[0085] Chemical name of compound of formula (3-1): 2-propyl-4,6-dihydrofuro[3,4-d]imidazole-4,6-dione.
[0086] The structural formula of the compound of formula (3-1) is as follows:
[0087]
[0088] Its preparation process: 106.0g (about 1.0mol) of powdered sodium carbonate and 119.0g (about 1.0mol) of newly distilled thionyl chloride and 500ml of anhydrous dichloromethane are stirred and mixed at room temperature, and 200.2 g (about 1.0mol) 2-propylimidazole-4,5-dicarboxylic acid (i.e. the compound of formula (3-0)) in 1000mL dichloromethane and dioxane (1:1 volume ratio) solution, add Reflux reaction for 3h. After cooling to room temperature, the mixture was filtered, the filter cake was washed with 200 mL of dichloromethane, the filtrates were combined, and the solvent was evaporated under reduced pressure to obtain 155.0 g of the compound of ...
Embodiment 3
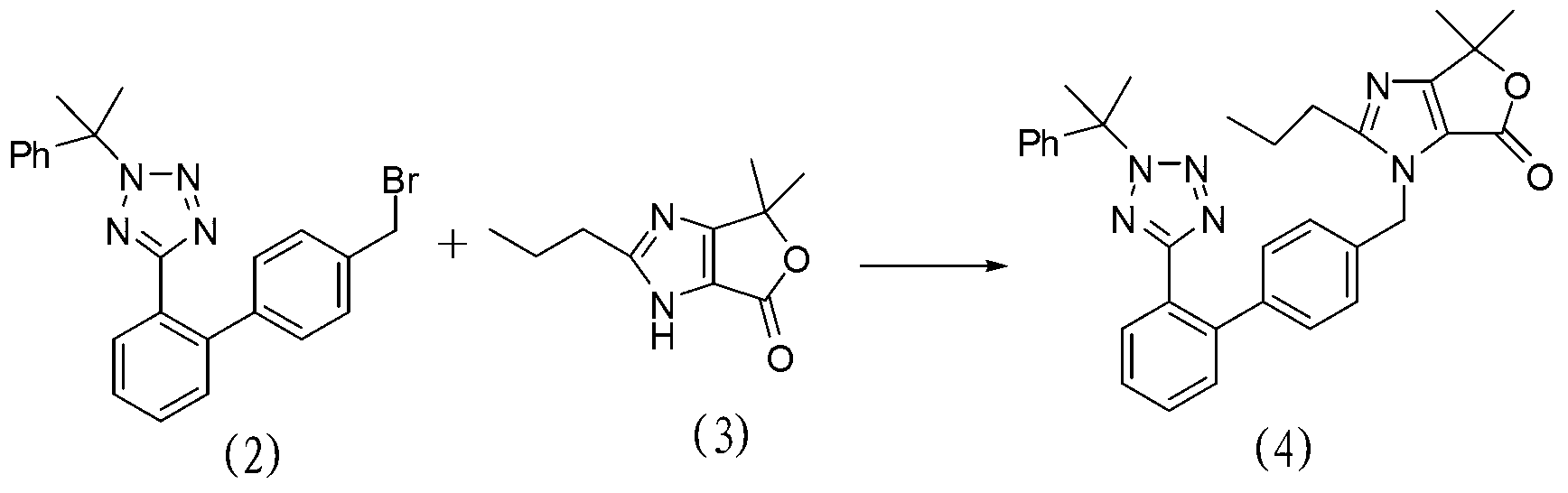
[0100] Embodiment 3: the preparation of formula (4) compound
[0101] The chemical name of the compound of formula (4): 4,4-dimethyl-2-propyl-1-[[2'-[2-(1-methyl-1-phenylethyl)tetrazole-5- Base]biphenyl-4-yl]methyl]-4,6-dihydrofuro[3,4-d]imidazol-6-one
[0102] The structural formula of the compound of formula (4) is as follows:
[0103]
[0104] Its preparation process: add 19.4g (about 0.10mol) of the compound of formula (3) to 200mL of acetone, stir, add 43.3g (about 0.10mol) of the compound of formula (2), 48.3g (about 0.35mol) of potassium carbonate, 1.6g (about 0.005mol) tetrabutylammonium bromide, heated to 50-55°C for 20 hours. Filter, wash the filter cake with 50 mL of acetone, evaporate the filtrate to remove the solvent under reduced pressure to obtain an oil, add 185 g of dichloromethane and 240 g of water, stir for 10 minutes, separate layers, and extract the water layer with 30 g of dichloromethane×2. The organic layers were combined, dried with anhydrous m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com