Medical application of ethane dimethane sulfonate (EDS)
A technology of ethane dimethanesulfonate and medicine, applied in the field of medicine, can solve the problems of narrow treatment range of EDS, limited application of EDS, etc., and achieve the effect of high safety and no systemic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: EDS was injected into the testicular artery to kill Leydig cells in rats
[0037] 1. 36 adult SD male rats. Grouped and randomly divided into 6 groups. In the first group, 6 rats were injected with 0.05ml vehicle (DMSO:H2O, 1:4, V / V) into the testicular artery. EDS was dissolved in vehicle, and the second group of 6 rats injected 0.05ml of 0.5mg / kg (mg / kg) EDS into the testicular artery; the third group of 6 rats injected 0.05ml of 2mg / kg EDS into the testicular artery; Four groups of 6 rats were injected with 0.05ml of 5mg / kg EDS into the testicular artery; 6 rats of the fifth group were injected with 0.05ml of 10mg / kg EDS into the testicular artery; 6 rats of the sixth group were injected with 0.05ml of testicular artery 15 mg / kg EDS; one EDS injection.
[0038] 2. After 7 days of intra-arterial injection of EDS, the rats were sacrificed by inhaling carbon dioxide.
[0039] 3. Immunohistochemical staining was performed on one testis of each animal after ...
Embodiment 2
[0044] Example 2: Rat intraperitoneal injection of EDS and testicular intraarterial injection of EDS potency comparison
[0045] 1. The other 36 adult SD male rats. Grouped and randomly divided into 6 groups. In the first group, 6 rats were intraperitoneally injected with 0.5ml vehicle (DMSO:H2O, 1:4, V / V). EDS was dissolved in vehicle, and 6 rats in the second group were injected intraperitoneally with 0.5ml of 10mg / kg EDS; 6 rats in the third group were injected intraperitoneally with 0.5ml of 20mg / kg EDS; 6 rats in the fourth group were injected intraperitoneally 0.5ml of 30mg / kg EDS; 6 rats in the fifth group were injected intraperitoneally with 0.5ml of 50mg / kg EDS; 6 rats in the sixth group were injected intraperitoneally with 0.5ml of 75mg / kg EDS; one EDS injection.
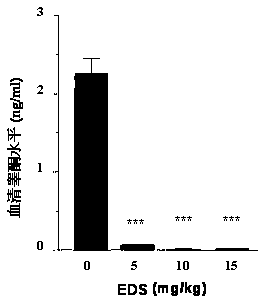
[0046] 2. Seven days after EDS injection, the rats were sacrificed by inhaling carbon dioxide. Blood samples were collected, centrifuged at 500 g, and serum was stored at -20°C for testosterone radioimmu...
Embodiment 3
[0049] Example 3: Comparison of the Sensitivity of EDS to Human and Rat Mesenchymal Cells
[0050] 1. The sensitivity of EDS to kill Leydig cells is species-dependent. For example, rat Leydig cells are sensitive to EDS but mouse Leydig cells are not sensitive (Rommerts F F. et al. 2004. J Endocrinol.181 :169-78). The sensitivity of human Leydig cells to EDS has not been tested. For our comparison the sensitivity of Leydig cells to EDS in human and rat testis.
[0051] 2. We established an in vitro culture system of seminiferous tubules, and human seminiferous tubules were derived from biopsy. Rat seminiferous tubules were taken from 90-day-old SD rats, and mouse seminiferous tubules were taken from 90-day-old C57BL / 6 mice. Human, mouse, and rat Leydig cells on the surface of the seminiferous tubules. We cultured human, mouse and rat seminiferous tubules in vitro according to the literature (Stanley E, et al. Endocrinology. 2012 Oct; 153(10):5002-10), and treated them with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com